The CARESCAPE R860 ventilator's backup batteries and replacement backup batteries were recalled as they were draining fast and could cause the device to shut down
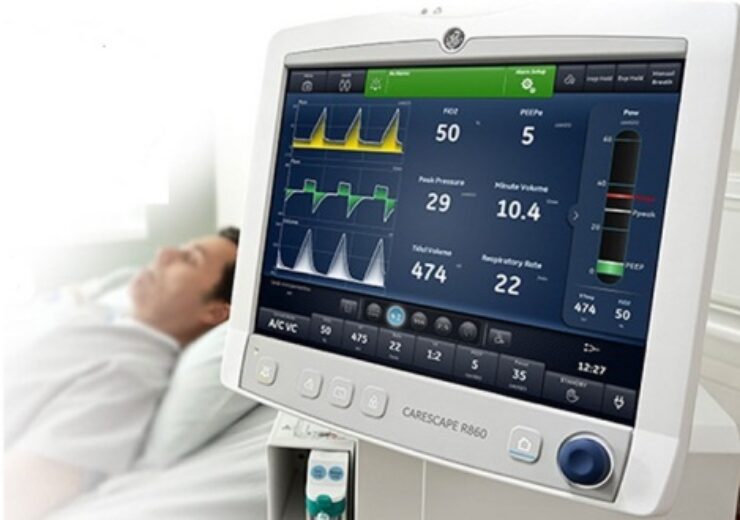
CARESCAPE R860, an intuitive Critical Care ventilator which uses advanced lung protection tools (Photo: Business Wire/GE Healthcare)
The US Food and Drug Administration (FDA) has classified the recall of backup batteries of GE Healthcare’s ventilators as a Class I recall, the most serious type of recall which could lead to injuries or death.
The CARESCAPE R860 ventilator’s backup batteries and replacement backup batteries were recalled as they were draining fast and could cause the device to shut down which will eventually prevent the patient from receiving breathing support.
The ventilator depends on the main AC power via a wall plug and backup battery in case the main power supply is absent such as during patient transport.
The firm also provides replacement batteries to use when the original backup battery expires.
FDA said that there have been 1,553 complaints associated with the use of the device. However, no injuries and deaths were reported.
GE Healthcare, the medical device-making division of General Electric (GE), started the recall of 4,222 of its ventilator batteries distributed between 2 April 2019 and 18 April 2022.
The company also issued an Urgent Medical Device Correction letter to its customers to conduct a battery performance test.
The firm suggested measures to check the backup batteries’ status and report to GE Healthcare with the acknowledgement response form.
FDA also instructed the health care professionals and customers to report the adverse reactions or quality problems of any medical device to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.
In May this year, FDA classified the recall of Avanos Medical’s Cortrak* 2 Enteral Access System (EAS) as a Class I recall.
