The percutaneous electrical nerve stimulator (PENS) system can be used for up to three days for symptomatic relief of post-operative pain following a C-section delivery
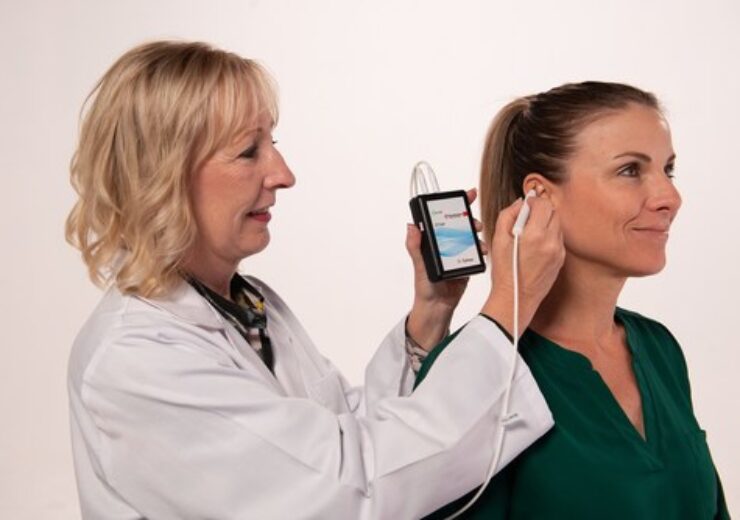
Demonstrating testing for placement of Primary Relief, a percutaneous electrical neurostimulation device cleared for treating pain following a Cesarean section (C-section. (Credit: PR Newswire/ DyAnsys Inc.)
DyAnsys, Inc., has announced that Primary Relief, a percutaneous electrical neurostimulation device, has been clearedto treat pain following a Caesarean-section (C-section).
The percutaneous electrical nerve stimulator (PENS) system can be used for up to three days for symptomatic relief of post-operative pain following a C-section delivery.
“This device has been shown to make a difference for patients, effectively relieving pain without reliance on opioids or other analgesics. This is a significant advancement in providing options to women,” said DyAnsys Chief Executive Srini Nageshwar. “We look forward to connecting with physicians and hospitals to providing this alternative to their patients.”
The wearable, battery-operated device is designed to administer periodic low level electrical pulses to the ear over 72 hours from the activation of the device. The electrical pulses are delivered to branches of the cranial nerves on the ear through a wire assembly and stimulation needles.
The effectiveness of the device was demonstrated in a single-center, double arm, randomized, controlled, parallel assignment prospective study involving 44 participants who underwent C-section delivery. The analysis showed that minimally invasive nerve stimulation intervention using Primary Relief device reduced the pain score faster than the standard analgesics. No additional opioid analgesics were required. No complications or adverse events were observed in any of the participants during the study period.
The nonclinical testing of Primary Relief device included biocompatibility testing, electrical safety (electromagnetic compatibility and safety), performance bench testing and software verification and validation.
DyAnsys offers two companion PENS devices – First Relief, with FDA clearance for the treatment of diabetic neuropathic pain, and Drug Relief, with FDA clearance as an aid to drug withdrawal.
Source: Company Press Release
