The firm’s OCTA blood vessel measurement technology will assist clinicians in early detection and management of diseases causing progressive blindness.
Optovue has also secured FDA approval for its three-dimensional projection artifact removal (3D PAR) software, which enables to enhance OCTA image quality.
The software also helps to accurately measure and interpret OCTA images.
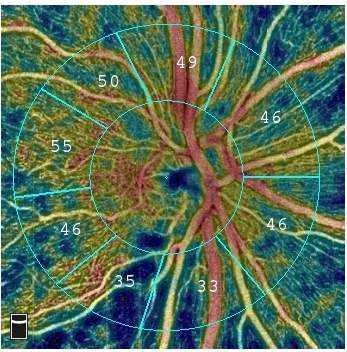
AngioAnalytics is said to contribute objective data and analysis to Optovue’s commercially available AngioVue OCTA technology, which offers high-resolution imaging of retinal blood vessels.
The combined technologies will help to develop color-encoded maps of the vessel densities of the retina or optic nerve.
They will also offer analyses of areas where there is blood vessel loss (non-perfusion), abnormal blood vessel growth (flow area), and various parameters to evaluate change to the foveal avascular zone, an area of the retina deeply affected by diabetic retinopathy.
AngioAnalytics software will also provide trend analysis, which enables physicians to objectively monitor retinal and vascular changes caused by disease progression or from treatment.
Optovue founder and CEO Jay Wei said: “We are thrilled to receive marketing clearance for our innovative AngioAnalytics technology as part of the AngioVue System to aid physicians in the early detection and management of sight-threatening eye diseases in the U.S.
“Analytic measurement tools will shift the treatment paradigm because they provide an objective measure of treatment efficacy, thereby enabling truly customized patient management and improved patient care.”
The company will exhibit AngioAnalytics on the AngioVue imaging system at the upcoming American Society of Retina Specialists meeting in Vancouver, Canada, which will be held between 20 and 25 July 2018.
Based in Fremont of California, Optovue is engaged in the advancement and commercialization of high-speed OCT and OCTA technology used to facilitate the diagnosis and management of eye diseases.






