The approval allows to offer both continuous and spot-check RRp in the US
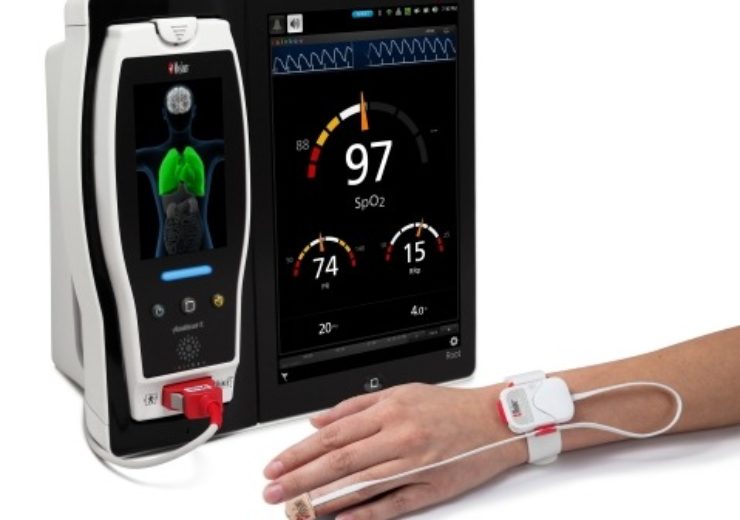
Masimo Root with SET, RRp, and Radius PPG (Credit: Business Wire)
Medical technology firm Masimo has secured approval from the US Food and Drug Administration (FDA) for its continuous RRp (respiration rate from the photoplethysmograph) monitoring of adult and paediatric patients with Rad-97, Radical-7, and Radius-7 Pulse CO-Oximeters.
The approval allows offering both continuous and spot-check RRp in the US, helping to support different pulse oximetry sensors and configurations, including the new non-cabled, tetherless, wearable Radius PPG.
The continuous RRp expands Masimo’s portfolio of respiration rate monitoring modalities
The continuous RRp expands Masimo’s portfolio of respiration rate monitoring modalities, which also included acoustic respiration rate (RRa) and NomoLine capnography (RRc).
Masimo’s RRp is said to be more suitable for use in lower acuity settings such as the general ward, where patients may not access required respiration rate monitoring technologies.
According to the company, the RRp is expected to play a significant role in supporting clinicians and public health officials to combat respiratory-related illnesses, including the coronavirus COVID-19, specifically when an additional sensor is not required.
Masimo provides a range of noninvasive and continuous monitoring technologies, including arterial oxygen saturation (SpO2), carboxyhemoglobin (SpCO), methemoglobin (SpMet), and total hemoglobin (SpHb), as well as spot-check thermometry with the non-contact infrared thermometer TIR-1.
The company offers a range of single-patient-use sensors to support these technologies and minimise the risk of patient-to-patient transmission of communicable diseases.
Masimo founder and CEO Joe Kiani said: “We aim to provide clinicians with the best monitoring tools so that they can provide the best care possible – which means recognizing that a monitoring method that works particularly well in one patient scenario may not be available or be the best choice in another.
“With the introduction of continuous RRp to our devices in the US, we are finally able to give clinicians in our home country a powerful third way to monitor respiration rate continuously, complementing other methods with a convenient and cost-effective single-sensor solution.”
In December 2019, Masimo secured FDA approval for its RD SET sensors with the company’s measure-through motion and low perfusion SET pulse oximetry.
