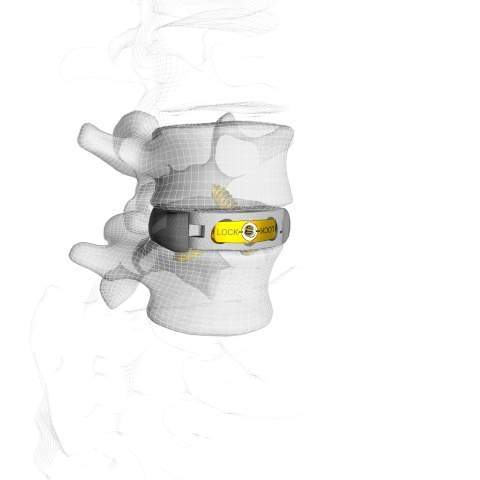
The M3 ALIF system is a sterile packed and integrated fixation device developed to be used in ALIF procedures. It features a universal bi-directional design with a simple and advanced locking mechanism.
CoreLink Surgical CEO Jay Bartling said: “M3 is our first stand-alone interbody fusion device to feature 3D printing – I continue to be impressed by the ability of our design teams to rapidly turn direct feedback and market demand into commercially competitive products.”
CoreLink has used its Mimetic Metal technology and patent pending StrutSure technology in the development of M3 ALIF system, enabling to create a combination of load-sharing support structure and interconnected lattice that minimizes material density for imaging.
According to the company, the CoreLink 3D printed ALIF portfolio, including both Foundation 3D ALIF and M3 ALIF, feature the largest footprint size available on the market, providing additional options for better anatomical fit.
CoreLink research and development vice president David Castleman said: “Our R & D team concentrated on creating a product that would feature our additive manufacturing capabilities, while also streamlining procedural efficiency. We’re extremely proud of the unique universal instruments offered with M3.”
CoreLink will showcase a full range of Foundation 3D products at the North American Spine Society’s annual meeting in Los Angeles, which will be held from 26 to 28 September.
Based in St. Louis of Missouri, CoreLink is known as The Source for Spine, and designs, manufactures and supplies a range of spinal implants and instrumentation.
The company’s product portfolio is comprised of pedicle screw systems, interbody spacers, plates, biologics and surgical instruments.
In August this year, CoreLink secured FDA 510(k) clearance for its Foundation 3D ALIF interbody device.
The Foundation 3D ALIF device features CoreLink’s Mimetic Metal technology, which helps to integrate crucial characteristics of natural bone with open-pore architecture and micro roughened porosity with significant hydro-wicking properties.


