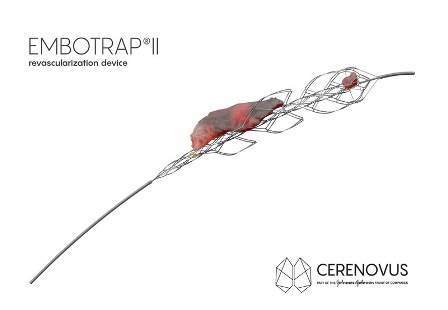
The next generation stent retriever will capture and remove life-threatening blood clots from the brain following an ischemic stroke.
The Embotrap II revascularization device will quickly restore blood flow by gripping and retrieving clots within the neurovasculature, enabling to reduce further complications.
The mechanical thrombectomy device will restore blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke within eight hours of symptom onset.
According to the company, the neurointerventional stroke physicians were able to restore blood flow in 80% of patients treated within three passes and in about half of patients within a single pass in the Arise II study.
The trial recruited 228 patients with large vessel occlusions and moderate to severe neurological deficits.
Embotrap II revascularization device will be used to treat patients, who are ineligible for intravenous tissue plasminogen activator (IV t-PA) or who fail IV t-PA therapy.
Arise II study lead author Dr Osama Zaidat said: “Mechanical thrombectomy with newer generation devices is increasingly becoming standard treatment for acute ischemic stroke.”
Embotrap II revascularization device was already approved in Europe, and used to treat more than 3,000 patients.
Cerenovus worldwide president Daniella Cramp said: “EMBOTRAP II is the product of deep collaboration between engineers and clinicians to better understand the science of blood clot, what causes them to form and how a mechanical thrombectomy device can interact with them to help improve outcomes.
“CERENOVUS is committed to advancing treatment with evidence-based solutions so that fewer and fewer people are affected by the ravages of stroke.”
Cerenovus provides a range of devices for the endovascular treatment of hemorrhagic and ischemic stroke.



