aScope Gastro is said to be the company’s first sterile single-use gastroscope that consists of new advanced imaging and design features in an integrated solution with next-generation display and processor technology
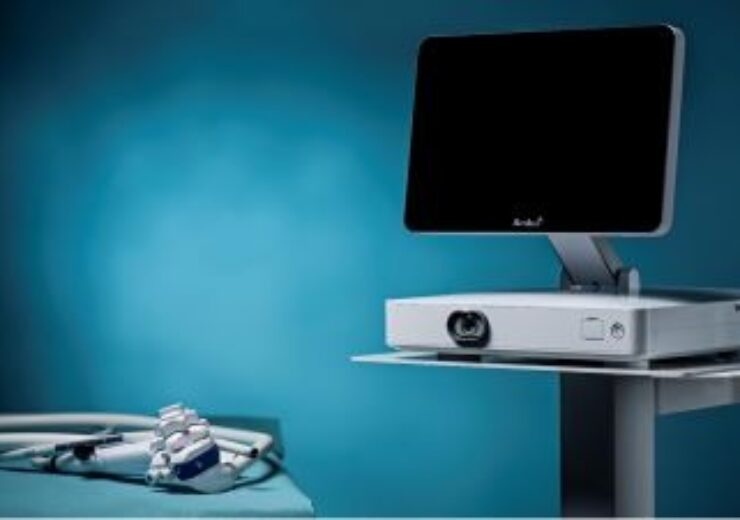
Ambu aScope Gastro and Ambu aBox 2 form a combined solution featuring a single-use gastroscope with a reusable display and processor unit. (Credit: Business Wire)
Denmark-based Ambu has secured 510(k) from the US Food and Drug Administration (FDA) for its single-use gastroscope and next-generation display unit.
aScope Gastro is said to be the company’s first sterile single-use gastroscope, which consists of new advanced imaging and design features in an integrated solution with next-generation display and processor technology.
According to the company, the aBox 2, with HD capabilities, will be a key part of Ambu’s endoscopy ecosystem.
The launch of aScope Gastro has enabled the company to enter the gastroscopy segment, where an estimated 20 million procedures take place per annum with reusable endoscope systems.
Ambu’s new solution allows customers to perform gastroscopies across a range of care settings, including endoscopy unit, OR (operating room), intensive care unit (ICU), emergency room (ER) and ambulatory surgery centre (ASC).
Ambu CEO Jose Gonzalez said: “The technology in our aScope Gastro and aBox 2 will set a new benchmark in terms of image quality and functionality and will power all of our next-generation launches.
“Our expansion within GI will extend Ambu’s position as the world’s most innovative single-use endoscopy player.”
The aScope Gastro is expected to help healthcare systems to reduce waiting lists and overcome staff shortages.
In addition, the sterile offering delivers a solution to the cross-contamination risks, specifically for vulnerable patients.
The single-use endoscopes company will now focus on the commercialisation of the aScope Gastro and aBox 2 in the US.
The aScope Gastro, along with the launch of the aScope Duodeno 1.5, is the company’s next step to expand into the GI segment. It will be followed by the launch of the next generation single-use duodenoscope called aScope Duodeno 2.0, colonoscope and cholangioscope.
In December last year, Ambu secured a contract Vizient, a healthcare performance improvement company in the US, for single-use visualisation devices.
