DiLumen Ik knife is part of a growing platform of accessories that work in conjunction with the second-generation DiLumen C2 EIP
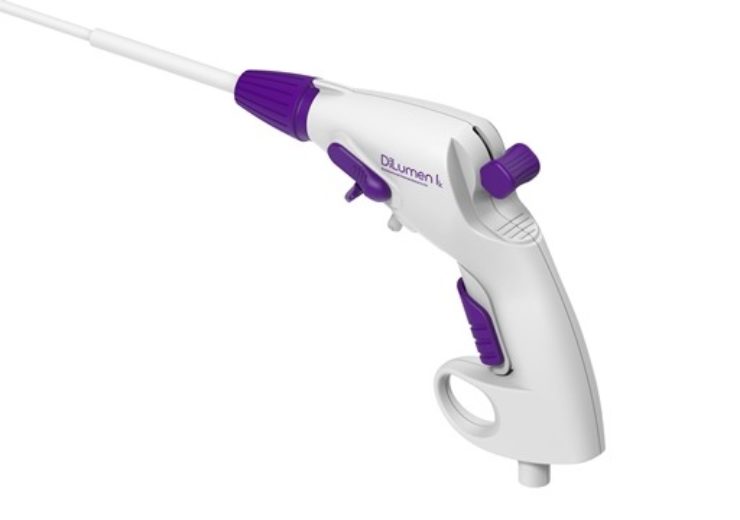
Image: DiLumen Ik endolumenal interventional knife. Photo: courtesy of Business Wire.
Medical device firm Lumendi has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its DiLumen lk endolumenal interventional knife.
DiLumen lk endolumenal interventional knife is a sterile, single-use and disposable monopolar electrosurgical device designed to be used for cutting, dissecting and cauterising tissue within the digestive tract during endoscopic procedures.
It will also help deliver a seamless submucosal injection for the creation and maintenance of a fluid cushion during tissue dissection.
DiLumen endolumenal interventional platform
According to the company, DiLumen endolumenal interventional platform (EIP) was used in more than 1,000 procedures by clinicians in the US and various EU countries such as Germany, France, Italy, Spain and the UK.
Three clinical studies with DiLumen EIP have also been completed, which further showed safety and cost effectiveness, said the company.
DiLumen lk knife is part of the accessories platform, which work in conjunction with the second-generation DiLumen C2 EIP.
The second-generation DiLumen C2 EIP has been developed to offer complete positioning of an endoscope in the large intestine, as well as aid with optical visualisation, diagnosis and endoscopic treatment.
Lumendi also provides other DiLumen accessories such as Ig endolumenal interventional grasper, which is a flexible endoscopic tool developed to grasp and manipulate tissue within the digestive tract under direct endoscopic visualization
Is endolumenal interventional scissors is a sterile, single-use and disposable monopolar electrosurgical device developed to be used for mechanical and electrocautery cutting, dissecting, and cauterising tissue within the digestive tract during endoscopic procedures.
Im endolumenal intervention mount is an ergonomically designed workstation designed to support various accessories and enable the clinician to conduct procedures in a comfortable standing or seated position.
Lumendi CEO Dr Peter Johann said: “We are responding to the needs of endoscopists by continuing to improve and develop the DiLumen platform of devices.
“In addition, we are also developing products and applications for the colon and the upper GI tract with the ultimate goal of reducing and replacing invasive open or laparoscopic surgical procedures.”
In May 2018, Lumendi secured FDA 510(k) clearance or its DiLumen Is endolumenal interventional scissors.
