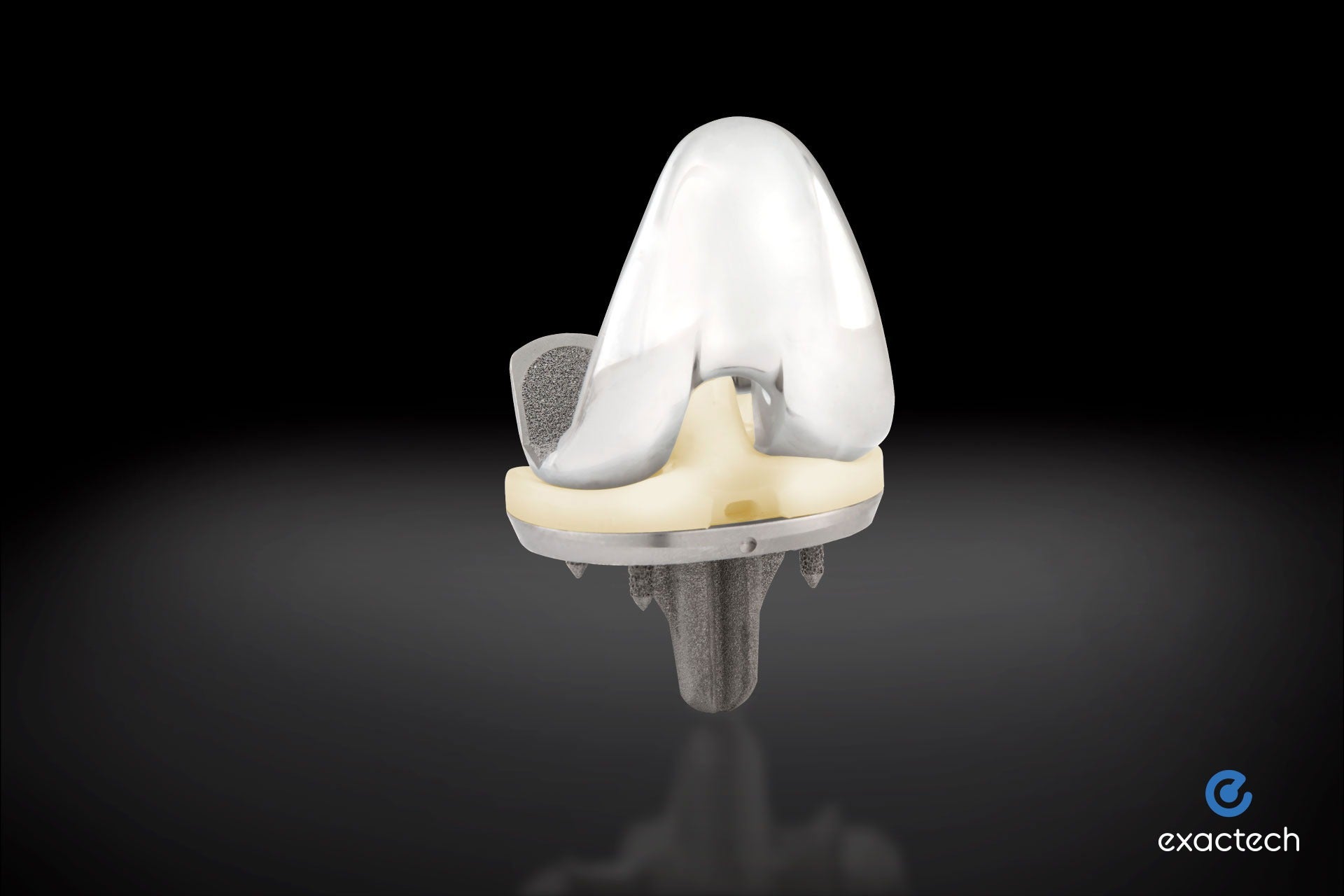
Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced the successful first surgery using its new, advanced Activit-E polyethylene for the Truliant knee replacement system.
Hany Bedair, M.D., performed the procedure at Massachusetts General Hospital (MGH) in Boston, where the material was developed by renowned polyethylene expert Orhun Muratoglu, Ph.D., director of the Harris Orthopaedic Laboratory at MGH. Dr. Muratoglu was also in attendance for the knee replacement case.
“It is a profound moment when you have the inventor who developed our advanced polyethylene in the case, performed at the hospital where it was developed,” said Adam Hayden, CMO and SVP, Large Joints Business Unit at Exactech. “Exactech is extremely proud to offer this next generation of highly crosslinked polyethylene with vitamin E antioxidant.”
Activit-E offers an optimized balance of material strength and toughness through chemically crosslinked polyethylene, while eliminating the need for gamma irradiation technology used in previous generations. It allows for active oxidative resistance and a long-term, high-performance polyethylene bearing, ultimately providing greater strength and active stabilization.
This new generation polyethylene is the latest, first-of-a-kind innovation by Dr. Muratoglu and his team, including Ebru Oral, Ph.D., director of biomaterials research. Muratoglu invented the first crosslinked polyethylene, and the first of multiple generations of vitamin E, antioxidant polyethylene for leading orthopaedic companies.
“It is exciting to perform the first surgical procedure with an implant material that was both conceived and developed within our hospital,” said Dr. Bedair. “With this advancement, we anticipate better outcomes compared to knees with traditional, highly crosslinked polyethylene.”
Activit-E was recently 510(k) cleared by the Food and Drug Administration for Exactech knee and ankle replacements. Activit-E will be showcased at the 2023 AAHKS Annual Meeting in Dallas, Texas, Nov. 2-5.
Source: Company Press Release




