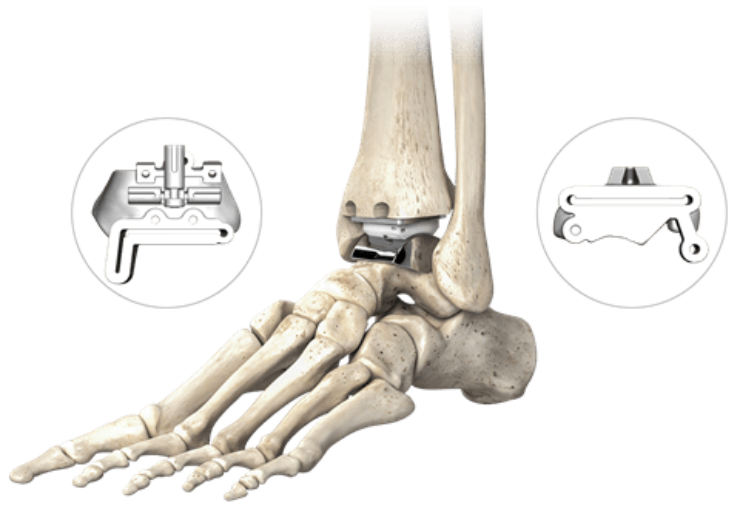
The STAR PSI system is designed to provide a personalised pre-operative plan for each patient and enable surgeons to receive and review a 3D visualisation of the patient’s ankle joint, including information about any defects
The STAR Patient Specific Instrumentation. (Credit: Enovis Corporation)
Enovis has received the US Food and Drug Administration (FDA) approval for Patient Specific Instrumentation (PSI) system for use with its STAR total ankle replacement system.
The US-based medical technology company designed the STAR PSI system to provide a personalised pre-operative plan for each patient.
The STAR PSI system enables surgeons to receive and review a 3D visualisation of the patient’s ankle joint, including information about any existing implants or bone defects.
It is intended for use with an updated and simplified surgical technique, which can reduce operative time during total ankle replacement cases, said the company.
Enovis foot and ankle president Gary Justak said: “We acquired the STAR Ankle just two years ago, and the FDA approval of STAR PSI is just one example of our team’s commitment and investment to taking this best-in-class product to the level it deserves.
“Surgeons now have leading pre-operative planning and cutting guides, that when paired with years of proven, clinical data, set STAR Ankle up for a brilliant future.”
Enovis is planning to commercially launch the STAR PSI system by the end of March 2023, with early product surveillance planned for this month.
The STAR PSI system is part of the company’s growing foot and ankle portfolio, which also includes other new solutions such as the DynaNail Helix, and Arsenal Ankle Plating System.
DynaNail Helix is designed to achieve sustained dynamic compression for subtalar fusion with an innovative, anatomically friendly design and simple, screw-like insertion.
Arsenal Ankle Plating System comes with 37 anatomically designed plates, which offer a plating solution for any ankle fracture.
Nevada-based foot and ankle orthopaedic surgeon Gregory Lundeeni said: “I’m excited about the Enovis addition of PSI technology which will have great benefit to STAR patients by providing personalised cutting guides and accurate implant sizing to match their unique anatomy.
“Surgeons will be able to take advantage of the improved precision and efficiency of STAR’s patient-specific cut guides.”
