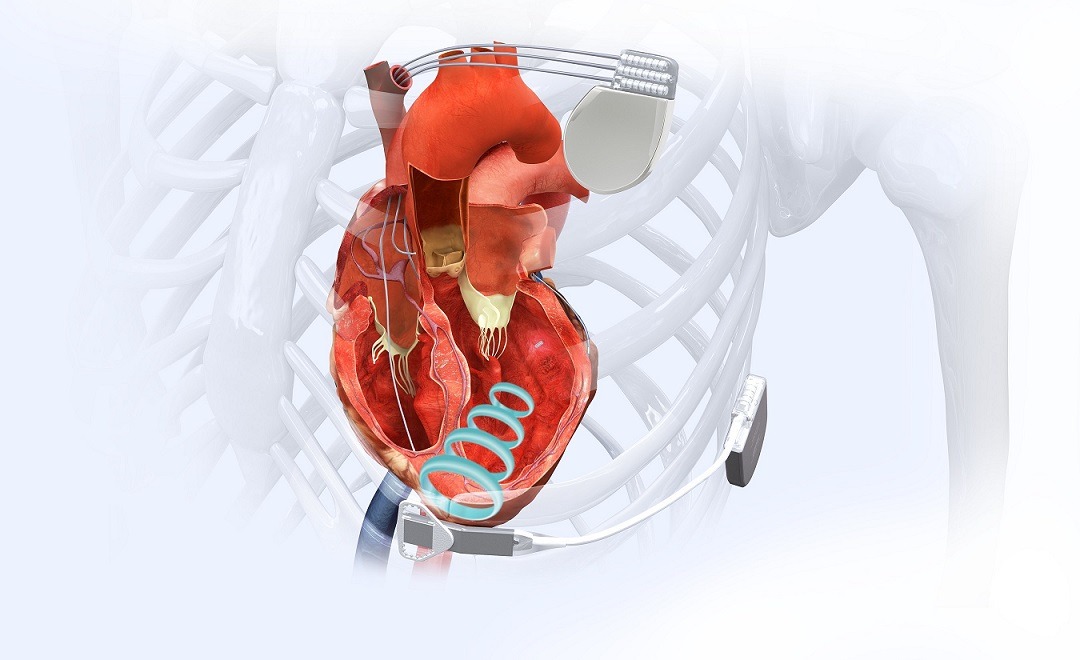
The company’s WiSE CRT System is claimed to be the world's only wireless, endocardial pacing system in clinical use for stimulating the heart's left ventricle
The WiSE Cardiac Resynchronization Therapy (CRT) System (Credit: EBR Systems)
Health tech start-up EBR Systems has secured $30m (£24.4m) funding to complete enrolment of a clinical trial to assess the safety of its wireless cardiac pacing system.
The funding will also be used for the commercialisation of the Wireless Stimulation Endocardially (WiSE) Cardiac Resynchronisation Therapy (CRT) System.
Led by Australian private equity firms Brandon Capital Partners and M.H. Carnegie & Co, the funding also saw participation from existing investors Split Rock Partners, Ascension Ventures, and Dr. Thomas Fogarty’s Emergent Medical Partners, among others.
The SOLVE CRT trial is the company’s multi-center, randomised, double blinded, prospective international study that will assess the safety and efficacy of the WiSE pacing technology.
The study is expected to support EBR in securing pre-market approval from the US Food and Drug Administration.
EBR Systems is expected to complete enrolment for SOLVE CRT in 2020. The study will enroll 350 heart failure patients who have failed to respond to, or are otherwise unable to receive, conventional CRT.
Brandon Capital Partners managing partner Chris Nave said: “We are encouraged by the results and patient benefit shown in previous studies using the WiSE System and as such, we are excited to be continuing to support EBR Systems to bring this important technology to the market.
“EBR’s technology promises to revolutionize cardiac resynchronisation therapy.”
EBR Systems’ wireless cardiac pacing system
Claimed to the world’s only wireless, endocardial (inside the heart) pacing system in clinical use, EBR Systems’ WiSE CRT System is intended to stimulate the heart’s left ventricle.
WiSE Technology facilitates cardiac pacing with a cardiac implant that is approximately the size of a grain of rice.
The system is designed to avoid the need for coronary sinus leads to stimulate the left ventricle in heart failure patients needing CRT.
EBR Systems has CE Mark approval for the WiSE CRT System, which is an investigational device in the US and not available for sale.
