Medical device company PQ Bypass has secured $60m in equity financing from Deerfield Management and existing investors Seroba Life Sciences and MedTech Venture Partners.
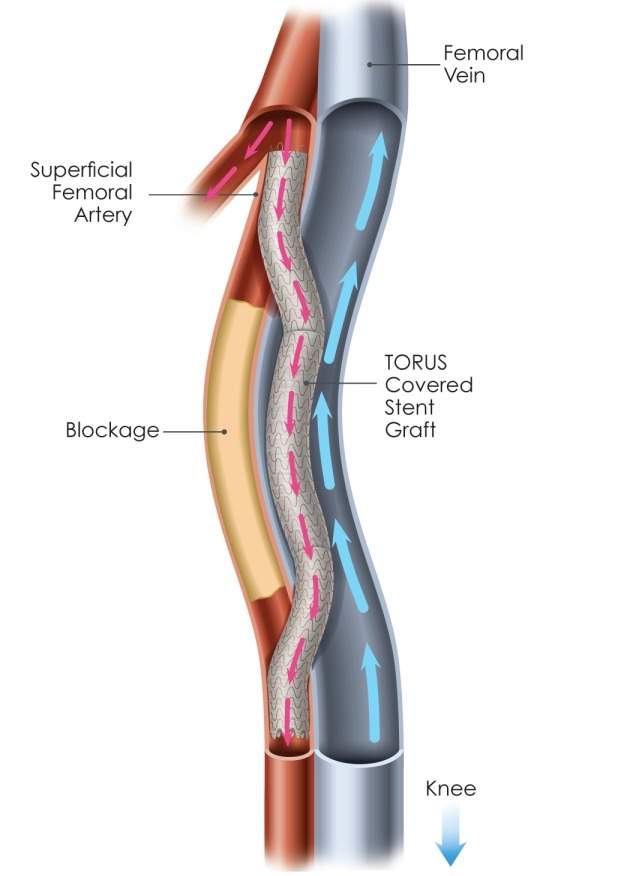
Image: Completed DETOUR procedure. Photo: Courtesy of Business Wire.
PQ Bypass stated that the funds will be used by the company to advance its technology for the treatment of long blockages in leg arteries caused by peripheral arterial disease (PAD).
In addition, the company will use the financing to convert nearly $15m in outstanding convertible debt and interest to equity.
PQ Bypass chairman and CEO Richard Ferrari said: “With this funding we continue our pursuit of the science that will bring us closer to commercial release of this important advancement in the care of patients with PAD.
“We are very fortunate to have Deerfield as an anchor investor and, together, we are working to develop a minimally-invasive treatment alternative for a disease that affects millions of people, worldwide.”
PAD is a common but serious circulatory condition wherein adequate blood flow does not reach the limbs due to a build-up of fatty deposits and calcium on the artery walls.
In people with PAD, leg arteries can be blocked by long segments of plaque that restrict blood flow to the lower leg and foot. This can result in pain, loss of mobility and even amputation. Extremely long blockages, such as those greater than 20cm can be quite challenging to treat.
Traditionally, physicians performed open bypass surgery, which was known to be durable but, it can give rise to several complications, lengthy hospital stays and prolonged rehabilitation.
Minimally invasive procedures such as angioplasty and stenting produce better results on shorter blockages. However, they have not been much effective on longer blockages, resulting in repeat procedures within one year.
Percutaneous femoropopliteal bypass (the DETOUR procedure developed by the company) is claimed to have been designed to offer durability of an open bypass with benefits of a minimally-invasive approach.
The procedure includes a stent graft technology developed by the company. This is placed from the superficial femoral artery, into the femoral vein, and back into the popliteal artery to create a detour around the blockage.
The stent graft bypass is claimed to re-direct oxygen-rich blood, with the goal of restoring blood flow to the lower leg and foot of the patient.
