Cutera, a provider of laser, light and other energy-based aesthetic systems, announced that Canada’s healthcare regulatory authority Health Canada has granted a Medical Device License for its Secret RF system.
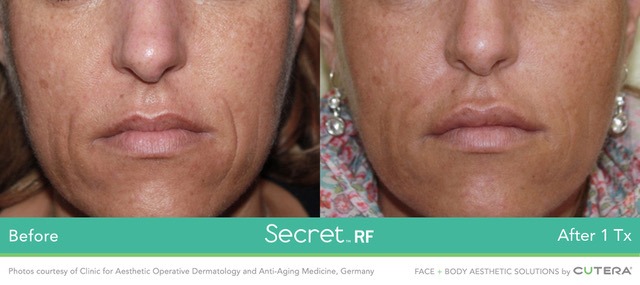
Image: Before and After 1 Treatment with Secret RF Microneedling System. Photo: Courtesy of Clinic for Aesthetic Operative Dermatology and Anti-Aging Medicine, Germany/Business Wire.
In January 2018, Cutera has launched the Secret RF system in the US, which is a novel fractional radio frequency (RF) microneedling system for tissue coagulation.
The system has been designed to stimulate and remodel collagen and address the common signs of aging and is useful for patients who wish to revitalize and refresh the appearance of their skin on all skin types.
Secret RF is capable of delivering energy at various depths, by adjusting the microneedles, customize the treatments to address each patient’s individual concerns such as fine lines, wrinkles, photoaging and striae.
Chicago-based Oak Dermatology has partnered with Cutera for several years and has performed many clinical studies with their research team for advancing aesthetic technology.
The Secret RF is expected to help Oak Dermatology expanded its capabilities to provide patients with individualized treatments, based on their skin concerns, skin type and lifestyle needs.
Cutera COO and Interim CEO Jason Richey said: “Canada is an important market for Cutera, and I am very happy that we are now able to introduce this innovative, skin revitalization technology to our Canadian customers.
“Secret RF’s microneedling technique is a very popular treatment method in the US, and offers excellent clinical results, for physicians and their patients looking to improve and revitalize skin concerns. With this approval, we look forward to positively impacting revenue in this market.”
The New Creation MediCosmetic at St. Catharines marks the first provider to offer Secret RF treatments in Canada using the Secret RF microneedling technology.
Dr. Sammy Kelada-Sedra of New Creation MediCosmetic said: “We are thrilled to pioneer this in Canada and extend our services beyond our practice across Ontario and other provinces. I look forward to helping my patients address even more of their dermal aesthetic concerns.”
The company referred that research shows the global aesthetic market was more than $11bn in 2018 and expected to grow at 11.1% per year through 2021, where skin revitalization procedures stood second in aesthetic procedure that consumers are considering, after non-invasive body shaping.
In September 2018, Cutera has received approval from Health Canada for its truSculpt iD non-surgical body contouring system.
The company said that the truSculpt iD has secured 510(k) clearance from the US Food and Drug Administration for non-surgical fat-reduction and circumferential reduction procedures.
