truSculpt is a non-surgical body sculpting treatment that uses monopolar radiofrequency technology
truSculpt iD body sculpting technology. Photo: courtesy of Business Wire.
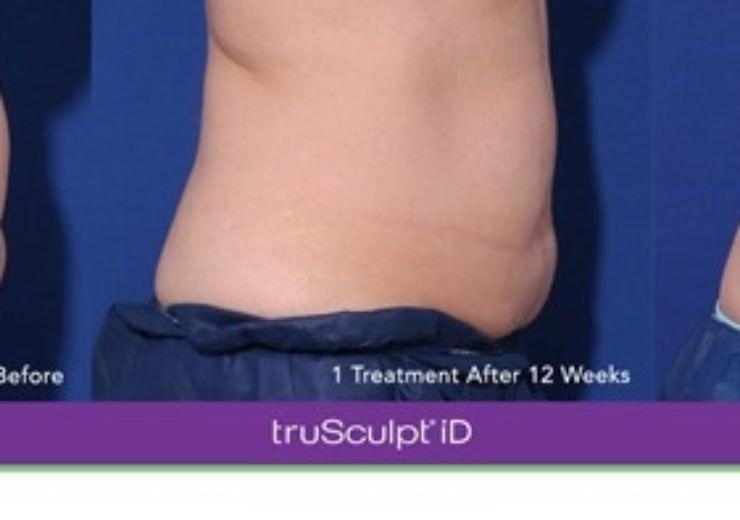
CUTERA, a leading provider of laser, light and other energy-based aesthetic systems for practitioners worldwide, announced today that Health Canada recently granted regulatory approval expanding the indications for use for truSculpt iD to include reduction of circumference of the abdomen and non-invasive lipolysis (the permanent removal of fat cells).
“Canada is a key market for Cutera and our full aesthetics technology product line,” said Cutera’s Chief Executive Officer, David Mowry. “truSculpt iD’s global popularity among providers and patients remains strong. This additional approval from Health Canada reinforces the acknowledgement of the clinical effectiveness for truSculpt iD to permanently eliminate fat.”
Research shows that noninvasive body sculpting is the #1 non-surgical fat reduction procedure, with a 14.5% increase year-over-year in body shaping procedures.1,2
The Company launched truSculpt iD in the US and Canada in 2018.
truSculpt is a non-surgical body sculpting treatment that uses monopolar radiofrequency (RF) technology. It is clinically proven to get rid of stubborn fat cells permanently by an average of 24%3, even in areas that are resistant to diet and exercise. truSculpt iD is clinically proven to eliminate fat in as little as one 15-minute hands-free treatment, completely personalized to an individual’s needs.
Brisbane, California-based Cutera is a leading provider of laser and other energy-based aesthetic systems for practitioners worldwide. Since 1998, Cutera has been developing innovative, easy-to-use products that enable physicians and other qualified practitioners to offer safe and effective aesthetic treatments to their patients.
Source: Company Press Release
