
As governments across the world attempt to halt the spread of Covid-19, many of their efforts are being hampered by shortages of crucial testing kits.
To combat this, the Food and Drug Administration (FDA) has granted an emergency use authorisation (EUA) for coronavirus tests in the US, relaxing many of the ordinary standards and allowing companies to gain regulatory approval for these kits more quickly.
Meanwhile, the UK government has called on large and small companies alike to help increase the country’s Covid-19 testing capacity.
With these resources, UK health secretary Matt Hancock has promised to boost the number of daily domestic tests to 100,000 by the end of April.
However, both the UK and US are still reportedly a long way behind the likes of Germany and South Korea in terms of patients being tested.
We take a closer look at the testing kits used to detect Covid-19, and why shortages of these critical pieces of equipment are becoming such a prominent issue in the response to the outbreak.
The different types of Covid-19 test
The wide range of testing kits currently being used in the Covid-19 pandemic can be divided into two main types — antigen tests, which detect if a patient is currently infected — and antibody tests, which reveal if the subject has previously had the virus.
Antigen tests — also referred to as nucleic acid tests, molecular tests or diagnostic tests — involve swab collection of a tissue sample from inside a person’s nose or mouth.
This sample is checked for the specific RNA (ribonucleic acid) or “genetic signature” of SARS-CoV-2 – the virus that causes Covid-19. The presence of this indicates that a person is currently infected.
Antibody tests, also known as serological tests, involve a blood sample being tested for specific antibodies.
The presence of such antibodies indicates a person’s body has produced the appropriate immune response to fight off the infection — which confirms they have previously been infected with Covid-19.
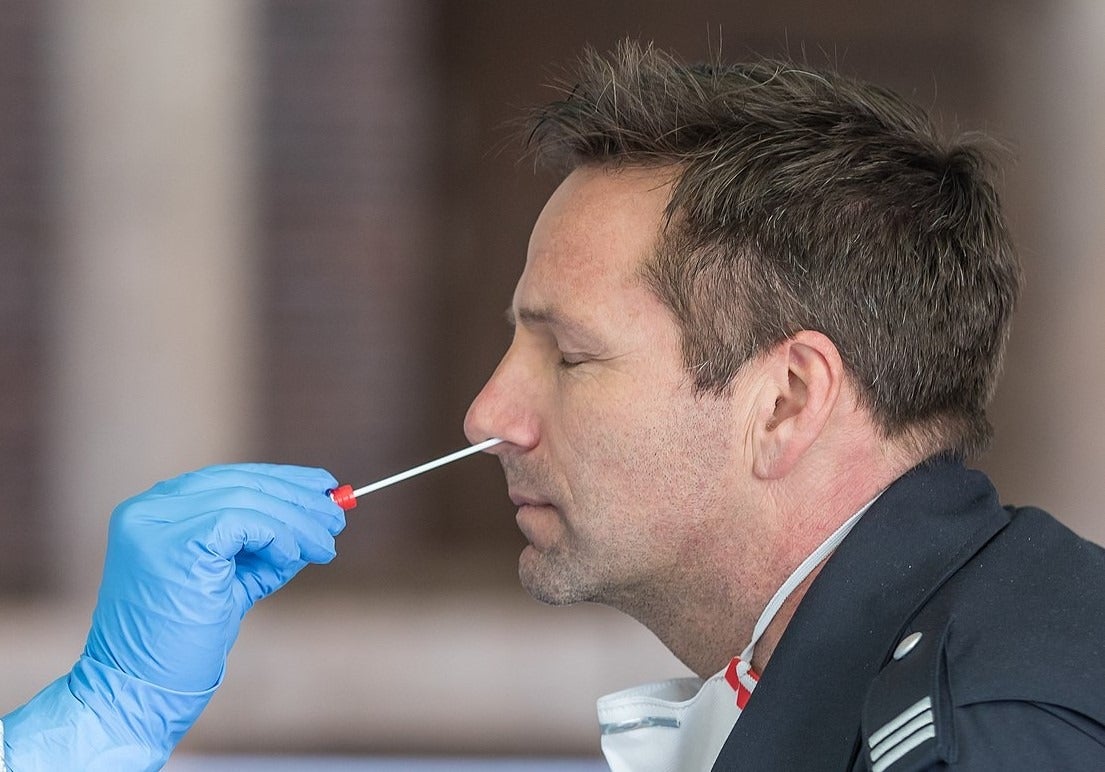
Antigen tests can be performed in a matter of hours, and are being used to diagnose patients and healthcare workers, alike. In the UK, they are currently only being made available to hospitalised patients experiencing severe symptoms of the virus.
Through February and March, the antigen tests were undoubtedly the priority as governments attempted to diagnose as many people infected with Covid-19 as possible.
However, antibody tests are now being viewed as increasingly important in both the US, UK and across Europe.
Not only can they be used to test asymptomatic carriers of Covid-19, helping experts ascertain the true spread of the disease, but antibody tests can also confirm that patients have built up immunity to the virus.
This could result in healthcare staff and other frontline workers avoiding lengthy isolation periods and returning to work. However, there are reports from China and South Korea of people being infected with the virus multiple times, meaning some patients may not be immune even after recovering.
Companies developing Covid-19 testing kits
Antigen tests
In the US, the first Covid-19 testing kit of any kind to be granted EUA was the Real-Time RT-PCR Diagnostic Panel, developed by the Centers for Disease Control and Prevention (CDC).
The diagnostic test, which uses DNA primers and probes to “flag up”, and detect, Covid-19 in a patient’s sample, was approved by the FDA on 7 February.
Swiss firm Roche announced its cobas SARS-CoV-2 test, which it claims produces results in as little as four hours, had been approved by the FDA on 12 March. The company said it would provide 400,000 of these testing kits per week in the US.
The test also received CE certification for use in the European Economic Area (EEA). This comprises all 28 EU member states — including the UK during its Brexit transition period — as well as Norway, Iceland and Liechtenstein.
Several American healthcare giants including Thermo Fisher Scientific, Abbott Laboratories and BD (Becton Dickinson) have developed testing kits under EUA guidelines too.

Abbott Laboratories’ product, in particular, has gained a lot of attention after the company said the molecular technology used in its test can detect negative results for Covid-19 in 13 minutes, and positive results in as little as five minutes.
Abbott announced its ID NOW test had been approved by the FDA on 27 March — making it the “fastest available molecular point-of-care test”. In a statement on 11 April, the company said it was producing 50,000 of the tests per day, and had shipped 566,000 of them across all 50 US states.
Several firms have also gained CE certification for their testing kits to be sold and used in the EEA — including US-based diagnostic company Co-Diagnostics, and French biotech group Novacyt.
On 6 March, UK diagnostic firm Mologic was given a £1m ($1.25m) grant by the government to develop a rapid diagnostic Covid-19 test. On 26 March, the company said it had started validating prototypes of its testing kit.
Antibody tests
Alongside the wide variety of the antigen tests produced to cope with shortages in the US, the first Covid-19 antibody test was also granted emergency use authorisation on 3 April.
North Carolina-based Cellex’s qSARS-CoV-2 IgG/IgM Rapid Test, which has also received CE certification, identifies immunoglobulin M and G antibodies in a patient’s blood sample.
The sample is then analysed in a lab, and the lab can get results from it in between 15 and 20 minutes, according to the US firm.
On 6 April, American healthcare organisation Mayo Clinic announced it had developed its own antibody testing kits and that it would attempt to carry out 8,000 tests per day across its laboratories.
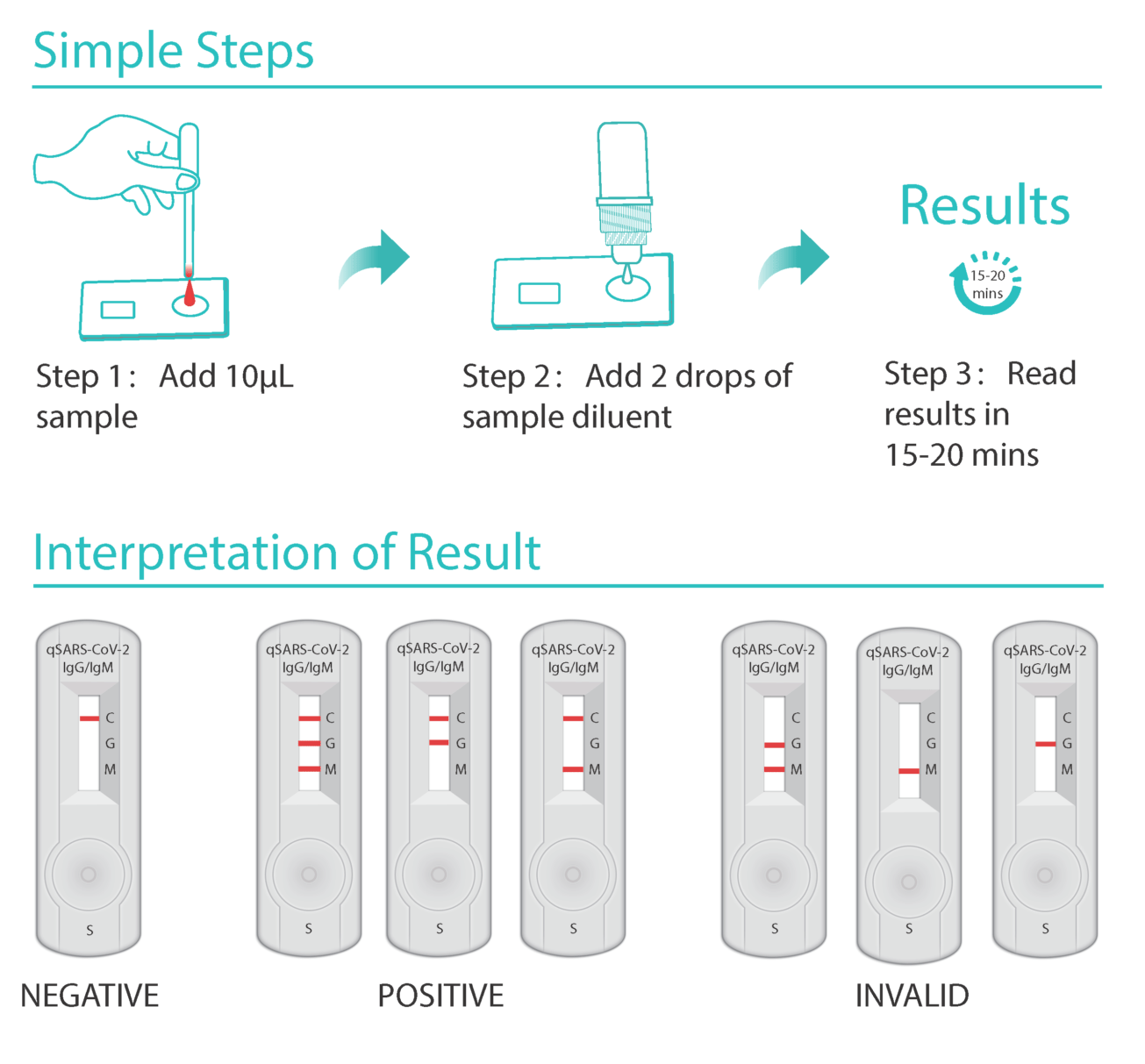
Later the same month, two more antibody tests — developed by US healthcare companies Chembio Diagnostics and Ortho Clinical Diagnostics — were given FDA approval under the EUA process.
There are also currently several of Chinese biotech firms that have been granted permission to distribute antibody testing kits across the US without gaining an EUA.
As of 15 April, only a handful of companies have been able to obtain a CE mark for their antibody tests.
This list includes Chinese firm Snibe Diagnostics, German manufacturer Euroimmun and Australian pharmaceutical company Beroni Group.
Medical equipment manufacturer SureScreen and Northern Irish biotech company Biopanda — both of whom claim their antibody test can return a result within 10 minutes — are among the UK firms to obtain CE certification.
What is in a Covid-19 testing kit?
Antigen tests
The majority of antigen tests being produced are referred to as “PCR tests” because they use a polymerase chain reaction (PCR) to detect the presence of the SARS-CoV-2 virus.
Several chemical components are needed for this process. Firstly, a chemical reagent is required to isolate and extract RNA from the patient’s sample.
Running the test itself also requires chemical elements called primers and probes, as well as two enzymes — reverse transcriptase and DNA polymerase — to detect the virus.
All of these biological molecules need to be manufactured in laboratories for use in antigen tests — and they must be manufactured under a certain level of quality control to ensure results are accurate.
Physical equipment, including a swab to collect samples, and a container to protect the sample while it is analysed, also forms part of an antigen test kit.
Because the samples collected need to be sent to labs for analysis, these diagnostic kits traditionally took longer out of the two types of diagnostic tool — with results taking up to a couple of days.
However, the rapid point-of-care kits that have been produced recently, such as Abbott’s, have cut this timeframe significantly.
Antibody tests
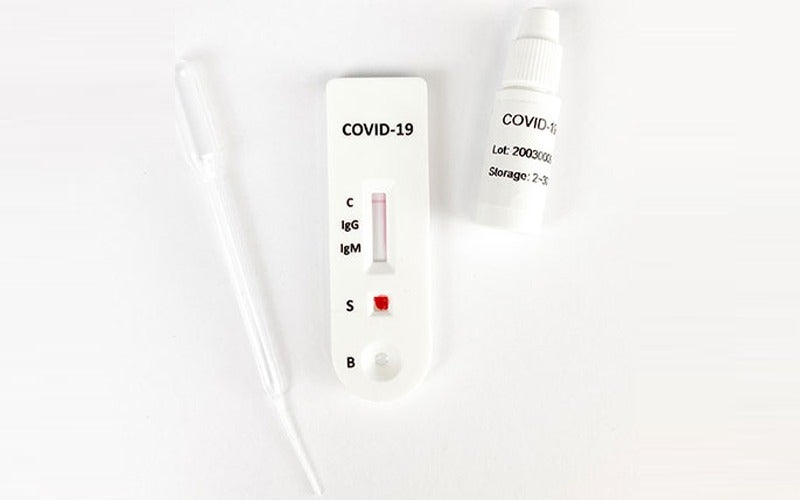
Antibody testing kits are essentially a device that pricks a patient’s finger — hence it often being referred to as the “finger prick” test — and takes a small sample of their blood.
A lancet is used to take the sample, which is then placed in the sample well of the testing kit.
A chemical known as a “buffer” is also added to the sample to help the blood flow along the test kit.
The test is a type of assay test, meaning it is designed to determine the components of a substance — in this instance, the presence of antibodies in blood.
The test strip inside the kit contains antibodies that will bind to specific biomarkers associated with Covid-19 — Immunoglobulin G (IgG) and Immunoglobulin M (IgM).
If a patient has previously contracted the virus, the two will bind and leave a visible test line — indicating a positive result for the virus. Only one of these biomarkers needs to be present to indicate a previous infection.
Because they consist of little more than a test stick, many antibody kits that have been receiving approval lately can show the result on the device itself — rather than needing laboratory analysis.
Current testing kits shortages
UK minister for the Cabinet Office Michael Gove has said huge demand for chemical reagents used in antigen testing kits explains current shortages.
In an attempt to rectify this, the government ordered 400,000 diagnostic kits from South Korea — a country lauded for its approach to testing, and dealing with the Covid-19 outbreak domestically.
But, on 4 April, The Guardian reported this order had been delayed because the testing kits had not yet been assessed by Public Health England (PHE).
Covid-19 antigen testing passed 10,000 people a day in the UK on 2 April, and while this increased to 15,000 as of 15 April, the country looks set to miss the government-set target of 100,000 daily tests by the end of the month.
Another setback occurred when 3.5 million antibody tests ordered from China were deemed unfit for widespread use by the UK’s Covid-19 testing chief, Dr John Newton.
At the start of April, Lawrence Young, professor of molecular oncology at Warwick Medical School, backed Gove’s claims by saying that “unprecedented demand” for all components of antigen testing kits — RNA extraction kits, primers and probes, and enzymes — was likely to be behind shortages in the UK.

He also cited a more “joined-up approach” thanks to national co-ordination via the Robert Koch Institute, along with the government’s relationship with the biotech industry, as the reason why Germany has fared better than anywhere else in Europe.
Similarly, in a World Economic Forum COVID Task Force meeting in March, South Korean foreign minister Kang Kyung-wha cited the fact his country had “acted early”, and accepted the importance of testing as soon as the outbreak hit South Korea.
Dr Alexander Edwards, an associate professor at the University of Reading, said shortages may be caused by a lack of physical equipment, such as swabs and containers, alongside insufficient amounts of the necessary chemicals.
The US has experienced similar problems, with underestimations of the importance of testing, coupled with manufacturing problems, previously cited as reasons.
On 10 March, CDC Director Robert Redfield told US media outlet Politico that a shortage of the reagents required to extract RNA from a patient sample during antigen tests may be the reason why the US has struggled to expand its testing capacity.
However, the US recently took steps to improve in this area — on 13 April, the Federal Emergency Management Agency (FEMA) announced the country would be receiving 750,000 testing kits from South Korea.
Problems with test accuracy
There are two elements to Covid-19 testing kit accuracy — specificity and sensitivity.
Specificity is a measure of how good a test is at telling you if you don’t have a disease, and as such, inaccurate results lead to “false positive” readings. False positives are patients who are detected as having Covid-19, when, in fact, they don’t.
Sensitivity is the opposite, as it indicates how good a testing kit is at detecting people who do have a disease. Problems with sensitivity lead to “false negative” readings – patients who are told they have not been infected when they have.
The main concern with antigen tests is “false negative” results.
Early data from research in China has suggested that common diagnostic testing kits may produce false negative results as much as 30% of the time.
The EUA process being used by the FDA attempts to solve this by requiring manufacturers to demonstrate their tests are 95% accurate in terms of sensitivity, and 100% accurate in terms of specificity.
The problem with this, as Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care, told US news outlet ProPublica on 10 April, is that these are being carried out on “contrived samples”.
This means they are not done using actual patient samples, and as such, physical problems with the test — such as the swab, the container, or the way the sample is taken — are not accounted for.
The antibody tests that are becoming increasingly sought after, alongside diagnostic tests, also need to achieve a certain level of accuracy to be considered suitable for widespread use.
It is thought that antibody tests must be 95% accurate — or more — in detecting whether a patient previously had Covid-19 to be considered suitable for widespread use in the UK.
This is because even a small percentage of false positive results may lead to people returning to work, and breaking social distancing protocols, because they wrongly believe they are immune to the virus, and therefore can’t be infected or spread it.
This led health secretary Hancock to suggest that “no test is better than a bad test”, which British publication The Times has speculated is the main reason why the 3.5 million kits ordered from China have not been deployed.
Data published recently in online medical journal The Lancet is limited, but suggests antibody tests may produce more of these false positive tests than antigen tests — but fewer false negatives.
While the UK has taken a cautionary approach up to now, the US, and the FDA, have been fast-tracking many antibody tests through the usual regulatory processes.
And while experts agree there is a definite need for these tests to properly assess the spread of Covid-19, as medtech journalist Elizabeth Cairns said on 6 April, an emergency authorisation for a testing kit does not amount to a determination by the FDA that a test has been proven accurate.
US healthcare giant BD was recently permitted to sell its antibody test in the US — despite BioMedomics, the company it partnered with to develop the kit, claiming its sensitivity accuracy is at 88.7% and its specificity accuracy stands at 90.6%.
The test has also received CE certification for use in the EEA, and BioMedomics said on 4 April it is already being sold in Italy — one of the countries worst affected by Covid-19.
Is there an end in sight for testing kit shortages?
With the Covid-19 outbreak currently heading for a peak in the UK and the US, both countries’ lack of preparation, and failure to prioritise critical widespread testing early on in the pandemic, has been brutally highlighted.
Conversely, South Korea had already tested more than 350,000 of its citizens by the end of March, and Germany was among the first countries to start developing a reliable test for the virus — the diagnostic it began producing in mid-January was quickly adopted by the World Health Organisation (WHO).
And, as many experts have already pointed out, not only have they both been able to “flatten the curve” by slowing the spread of the virus domestically, but the Covid-19 death rate is also noticeably lower in South Korea (2%) and Germany (3%) than it is in the US (5%) and the UK (12%).
Currently, attempts by the US and the UK to elevate their testing capacity are being hampered not only by shortages of key ingredients and physical parts, but also by governments in both countries failing to co-ordinate testing protocols until after the virus had spread widely within their populations.
Recent concerns around the accuracy of novel antibody tests — and the ramifications of putting them into use without validating them — present yet another barrier with no clear solution.
And as worldwide cases surpass two million, with close to 150,000 deaths, the current challenges surrounding testing kits seem to be the same as those anticipated by Roche CEO Severin Schwan almost exactly a month ago, on 19 March, during a Covid-19 press briefing.
“The demand is by far outstripping the supply”, he said.
“So, it is very important in my view, and I speak here on behalf of many experts, that testing is really targeted for high-risk patients and patients who show signs and symptoms of the disease.
“Broad-based testing at this stage is simply not feasible.”




