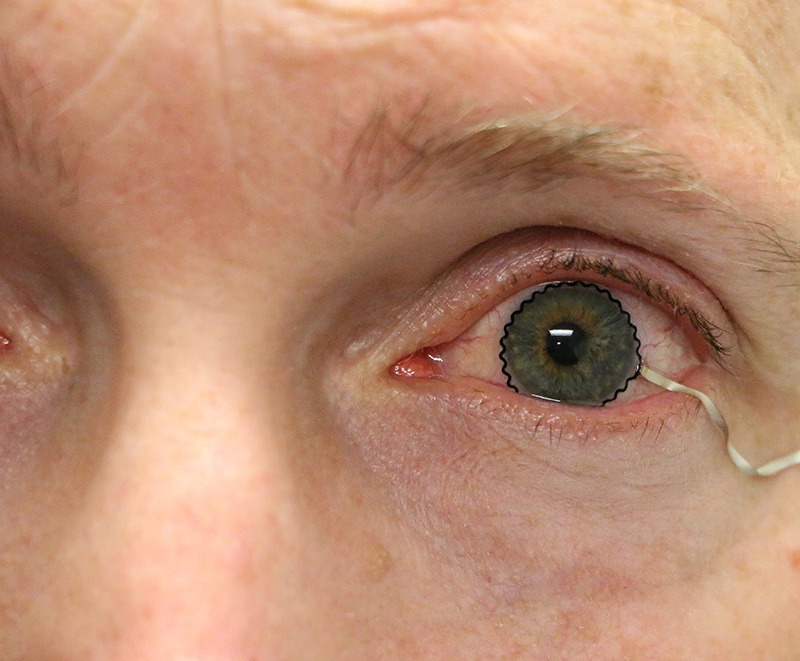
A team of researchers from Purdue University have enabled commercial soft contact lenses to be a bioinstrumentation tool to the detect, diagnose and monitor eye disease.
Patients typically undergo an electroretinogram involving the use of topical anaesthetic and a speculum to test the function of the retina at the back of the eye and determine whether they have one of several disorders.
But the Purdue team’s work, published in the journal Nature Communications, has given pre-clinical validation to a technique that uses soft contact lenses to negate the need for anaesthesia or a speculum in the process.
Chi Hwan Lee, the Leslie A. Geddes assistant professor of biomedical engineering and assistant professor of mechanical engineering at Purdue who led the development team, said: “This technology will be greatly beneficial to the painless diagnosis or early detection of many ocular diseases, including glaucoma”
“Since the first conceptual invention by Leonardo da Vinci, there has been a great desire to utilise contact lenses for eye-wearable biomedical platforms.”
Sensors or other electronics previously couldn’t be used for commercial soft contact lenses because the fabrication technology required a rigid, planar surface incompatible with the soft, curved shape of a contact lens.
But the Purdue researchers have patented a way to enable the integration of ultra thin, stretchable biosensors with commercial soft contact lenses via wet adhesive bonding, and is now in the market for a commercial partner to market the technology.
The biosensors embedded on the soft contact lenses record electrophysiological retinal activity from the corneal surface of human eyes, without the need for the topical anaesthesia that has been required in current clinical settings for pain management and safety.
The Purdue research team has partnered with the Borish Center for Ophthalmic Research at Indiana University, and has stated that their technology will be ready for clinical trials to be run in accordance with the institution soon.
The Center’s director and prospective leader of the trials professor Pete Kollbaum said: “This technology will allow doctors and scientists to better understand spontaneous retinal activity with significantly improved accuracy, reliability, and user comfort.”


