The CONFORM study is said to support the US FDA approval of the company’s CLAAS implant for left atrial appendage occlusion (LAAO) and stroke prevention
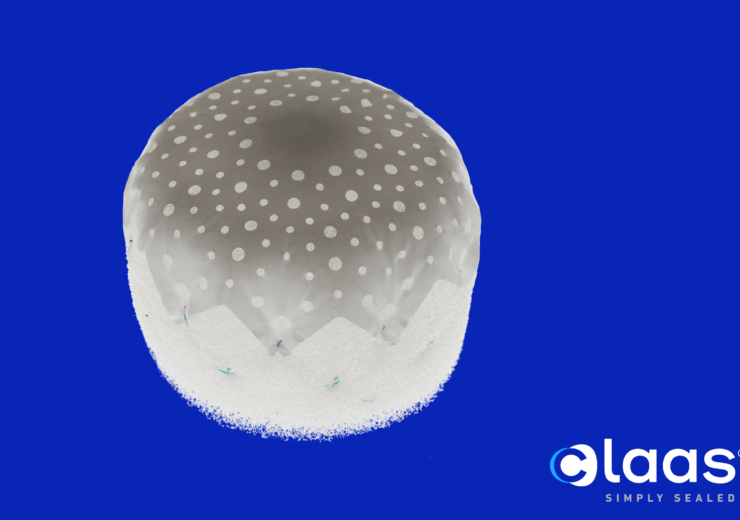
CONFORM trial will support US FDA approval of CLAAS System. (Credit: Conformal Medical, Inc.)
Conformal Medical has started recruiting patients for the investigational device exemption (IDE) study of its CLAAS System, dubbed CONFORM, at two sites in the US.
The IDE study will evaluate the safety and efficacy of the company’s CLAAS System compared to commercially available LAAO devices.
Conformal Medical said that the CONFORM trial would support the US Food and Drug Administration (FDA) pre-market approval of its device.
The first patients were treated by Heart & Vascular Institute executive director Dr Shephal Doshi, and Lankenau Institute for Medical Research professor Dr William Gray, who were co-principal investigators of the CONFORM trial.
Dr Gray said: “In this study, we will be evaluating the novel foam-based design of the CLAAS implant against the existing FDA-approved devices to evaluate performance on several metrics, including procedural safety, completeness of seal and incidence of device-related thrombus.”
Dr Doshi said: “Based on our experience in the Early Feasibility Study, the CLAAS System is highly conformable to accommodate different anatomies. The study is designed to demonstrate the benefits of this technology for both implanting physicians and patients.”
The multi-centre, randomised controlled IDE study is designed to enrol nearly 1,600 patients across the US, Canada, and Japan.
As part of the early feasibility studies, its CLAAS System has been successfully implanted in more than 75 patients, said the company.
The US-based medical equipment supplier is focused on next-generation Left Atrial Appendage Occlusion (LAAO) technology.
Its CLAAS System is designed to seal the LAA in patients with non-valvular Afib to reduce the risk of stroke without the need for anticoagulants.
The device features unique foam-based architecture, and the implant addresses a wide spectrum of LAA anatomies with only two sizes, said the company.
The system is said to simplify the delivery and eliminate the need for a procedural transoesophageal echocardiogram, enabling physicians to perform the procedure without general anaesthesia.
Conformal Medical president and CEO Andy Levine said: “We are pleased to enrol the first patients in the CONFORM trial, an important milestone for the company. Kicking off the study builds on the strong momentum from our EFS experience.
“This IDE includes the rigour of both a randomized study comparing CLAAS to commercial devices and a separate sub-study designed to support a conscious sedation, ICE-driven approach; a critical step towards our goal to transform LAAO and reduce the risk of stroke, without the need for anticoagulants.”
