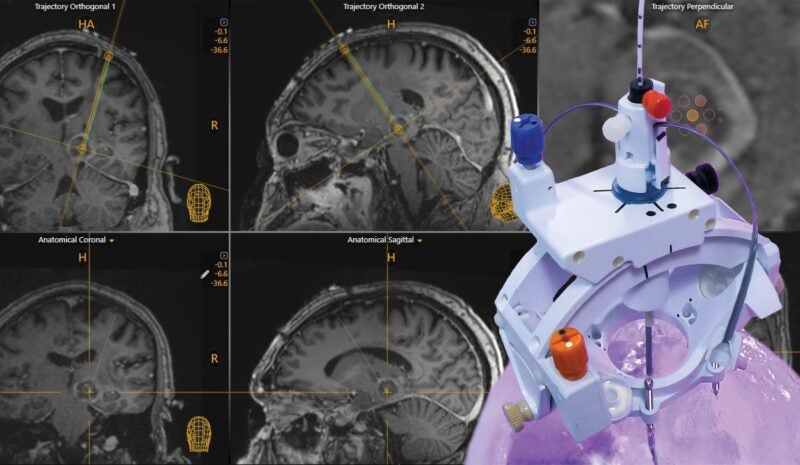
ClearPoint Neuro said that its ClearPoint Prism Neuro Laser Therapy System has demonstrated accuracy as per the preclinical results of an in vivo validation study.
The Prism System is said to offer the only non-cooled neurosurgical laser applicators on the market. Its next-generation laser applicator technology removes the need for external cooling, said ClearPoint Neuro.
The system is currently in limited market release at a few university medical institutes in the US.
The results, published in the Journal of Neurosurgery, showed that the brain tissue temperature can be measured with the Prism System with a mean absolute error of less than 1°C in almost real-time.
In addition, there was a strong correlation between the histology and the lesion’s morphology as shown by Thermoguide MR-thermometry software.
ClearPoint Neuro and medical device company Clinical Laserthermia Systems (CLS), its Swedish partner, carried out the study under the direction of principal investigator John Rolston.
The study’s objective was to assess the Prism System’s accuracy, safety, and effectiveness.
This was accomplished using survival histology to demonstrate a predicted safety margin and identifying thermal damage thresholds (TDTs) based on said histology that best predicts irreversible tissue damage.
The trial also assessed the precision of temperature prediction in comparison to real temperature changes in vivo.
In addition, the Prism System has been used in conjunction with the company’s recently US Food and Drug Administration (FDA)-approved Array software version 1.2 to increase the practicality of neuro laser therapy.
ClearPoint Neuro laser therapy global segment leader director Chris Osswald said: “This robust, peer-reviewed validation demonstrates that the advantages of Prism result in excellent predictability of targeted cell death.
“Our comparison of histopathology to damage estimation is arguably the most definitive test we could have performed and was part of the dataset that led to FDA clearance.”
The ClearPoint Prism Neuro Laser Therapy System was cleared by the FDA in September 2022.



