OrSense NBM 200 is a non-invasive medical device to measure pre-donation hemoglobin levels without using a needle or finger prick
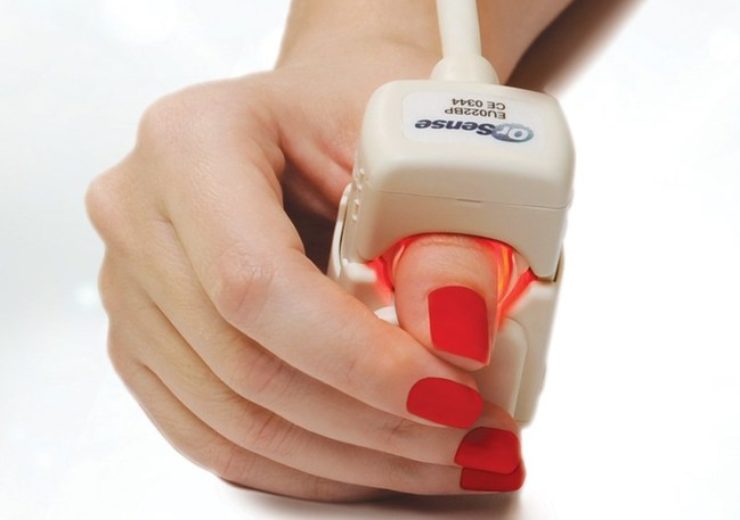
Image: The OrSense NBM-200 is painless and does not require the use of any disposable supplies. Photo: Courtesy of OrSense Ltd.
OrSense, a leading developer of systems for non-invasive measurements of hemoglobin, announces the implementation of its NBM-200 non-invasive hemoglobin measurement device by Children’s Hospital Los Angeles (CHLA).
CHLA is using OrSense NBM 200, a non-invasive medical device that measures pre-donation hemoglobin levels without using a needle or finger prick, to enhance its donors’ blood donation experience. Unlike traditional hemoglobin testing methods, the OrSense NBM-200 is painless and does not require the use of any disposable supplies, reducing biohazardous waste handling.
“Across the U.S., we see a growing number of requests for our non-invasive device, and we are thrilled to have this business partnership with Children’s Hospital Los Angeles. Our technology allows the blood donors to be more comfortable during the pre-donation process and provides for more efficient, lower-cost blood center operations,” said Chip Neff, President of OrSense U.S.
“At Children’s Hospital Los Angeles, we are committed to using innovation to improve our patient and visitor experience,” said Steven Gregurek, MD, Medical Director of Transfusion Medicine at Children’s Hospital Los Angeles. “Eliminating the need for a needle or finger prick is a welcome advance that we can now offer our blood donors.”
The NBM-200 was recently cleared by FDA’s CBER division for use in U.S. blood donation centers. Children’s Hospital Los Angeles is the first on the West Coast to implement this technology.
Source: Company Press Release
