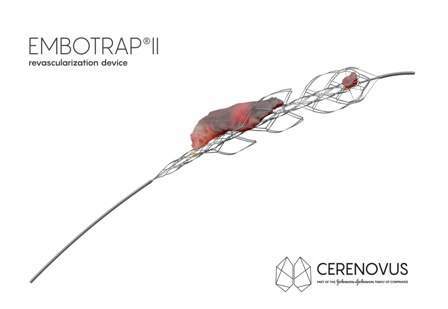
The first patients have been treated with the Embotrap II, which is a next generation stent retriever used to capture and remove life-threatening blood clots from the brain following an ischemic stroke.
In May this year, Cerenovus secured approval from the US Food and Drug Administration (FDA) for Embotrap II to commercialize the mechanical clot removal or thrombectomy device.
Featuring dual-layer design, the Embotrap II device will engage and grip stroke-inducing blood clots differently compared to other stent retrievers, enabling doctor to maintain engagement and control of the clot with minimal compression during removal.
In the ARISE II (analysis of revascularization in ischemic stroke with Embotrap device) study, the neurointerventional stroke physicians restored blood flow in 80% of patients treated within three passes and in around half of patients within a single pass.
The study enrolled 228 patients with large vessel occlusions and moderate to severe neurological deficits.
Cerenovus worldwide president Daniella Cramp said: “While most devices are designed on the characteristics of retrieval, the EMBOTRAP II Device was designed based on extensive research into the characteristics of clot. This led to its innovative dual-layer design, which is unlike any other stent retriever in the U.S. or Europe.
“CERENOVUS is committed to advancing treatment with evidence-based solutions that help improve outcomes for stroke patients.”
The company also secured approval for Embotrap II device in Europe, and is used to treat more than 5,000 patients in the region.
Embotrap II revascularization device has been developed to restore blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke within eight hours of symptom onset.
According to the company, patients who are ineligible for intravenous tissue plasminogen activator (IV t-PA) or who fail IV t-PA therapy are candidates for treatment.
Cerenovus provides a range of devices used in the endovascular treatment of hemorrhagic and ischemic stroke.






