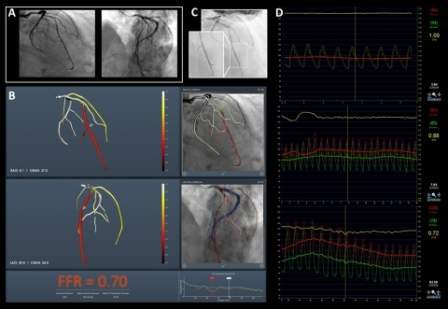
CathWorks’ FFRangio system is a non-invasive FFR platform, which rapidly and precisely delivers objective multi-vessel physiologic measurements to cost-effectively optimize and confirm intraprocedural percutaneous coronary intervention (PCI) therapy decisions.
The system has been developed to deliver the objective FFR guidance required to optimize PCI therapy decisions for every patient.
The study has showed that the sensitivity and specificity of the CathWorks FFRangio system were 93.5% and 91.2%, while diagnostic accuracy was 92% overall.
According to the company, the CathWorks system can significantly increase physiologic coronary lesion assessment in the cath lab, enabling to improve patient outcomes.
University of Erlangen medicine professor and FAST-FFR trial leading enroller Dr Stephan Achenbach said: “Guidelines mandate that revascularization decisions are based on the presence of ischemia. However, not every stenosis or invasive angiography, even if perceived as ‘severe,’ causes ischemia.
“Equally important, lesions that do not appear severely stenotic may cause ischemia at times and may hence benefit from revascularization. Invasive angiography in such cases is incomplete without assessment of ischemia.”
The 19 on-site cath-lab clinicians measured the data of the FFRangio system through blinding to the invasive FFR measurements.
Angiograms have been captured by dozens of operators at ten hospitals with the support of major angiography systems from Siemens, Phillips, Canon, and GE.
Stanford Medical Center cardiology professor and trial principal investigator Dr William Fearon said: “The results of the FAST-FFR trial demonstrate a very high accuracy and strong correlation between the reference standard, pressure wire-derived FFR and FFRangio.”
CathWorks FFRangio system is currently under development, and is not yet secured approval from the US Food and Drug Administration (FDA).
In August this year, the company submitted the system to the FDA for review and 510(k) market clearance.
CathWorks expects to receive 510(k) market clearance by the end of this year.
CathWorks is involved in applying its advanced computational science platform to optimize PCI therapy decisions and elevate coronary angiography from visual assessment to an objective FFR-based decision-making tool for physicians.




