The FDA approval is based on results of a two-year Investigational Device Exemption (IDE) clinical study, in which implant showed superiority over microfracture and debridement, the current Surgical Standard of Care (SSOC) in treating knee joint surface lesions, chondral and osteochondral defects
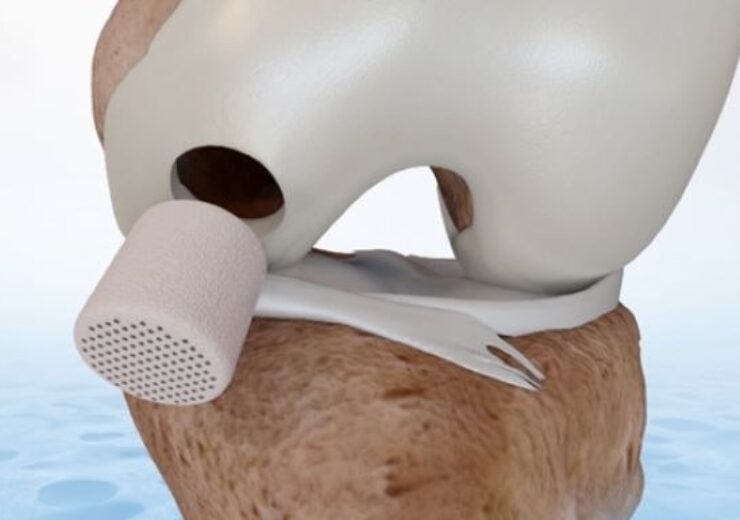
CartiHeal’s implant for cartilage and osteochondral defects. (Credit: CartiHeal Ltd.)
Israeli medical device maker CartiHeal has received the US Food and Drug Administration (FDA) premarket approval (PMA) for its Agili-C implant to treat certain cartilage and osteochondral defects.
The implant is indicated for knee-joint surface lesions, measuring International Cartilage Repair Society (ICRS) grade III or above, with a total treatable area of 1-7cm2, without severe osteoarthritis.
Agili-C is a cell-free, off-the-shelf implant intended for the treatment of cartilage and osteochondral defects in traumatic and osteoarthritic joints.
It is a porous, biocompatible, and resorbable bi-phasic implant, which contains interconnected natural inorganic calcium carbonate (aragonite).
In October 2020, the US FDA has granted breakthrough device designation for the Agili-C implant to treat cartilage lesions in arthritic and non-arthritic joints.
CartiHeal founder and CEO Nir Altschuler said: “The 2-year study results, which demonstrated superiority of the Agili-C implant over the current surgical standard of care, offers an important potential benefit to millions of patients.
“This milestone achievement was made possible due to the support of our regulatory advisors, Hogan Lovells, our statistical consultants, Biomedical Statistical Consulting, and the many dedicated investigators and patients who participated in our studies.”
The FDA approval of Agili-C implant was supported by the results from a two-year Investigational Device Exemption (IDE) study.
The trial enrolled 251 subjects at 26 sites in the US and abroad, and randomised them in 2:1 ratio, with 167 in the Agili-C arm and 84 in the SSOC arm.
In the study, the implant showed superiority over microfracture and debridement, the current Surgical Standard of Care (SSOC) in treating knee joint surface lesions, chondral and osteochondral defects.
The primary endpoint of the study was the change in the average Knee injury and Osteoarthritis Outcome Score (KOOS Overall), from baseline to 24 months.
The score contained five subscales, including pain, other symptoms, quality of life (QOL), activities of daily living (ADL) and sports.
Agili-C showed a degree of improvement that was similar for subjects with Mild-moderate Osteoarthritis, and with Large Lesions, compared to SSOC, said CartiHeal.
