The European Commission has granted a total of €33m (£29m) funding to CARMAT to develop the artificial heart
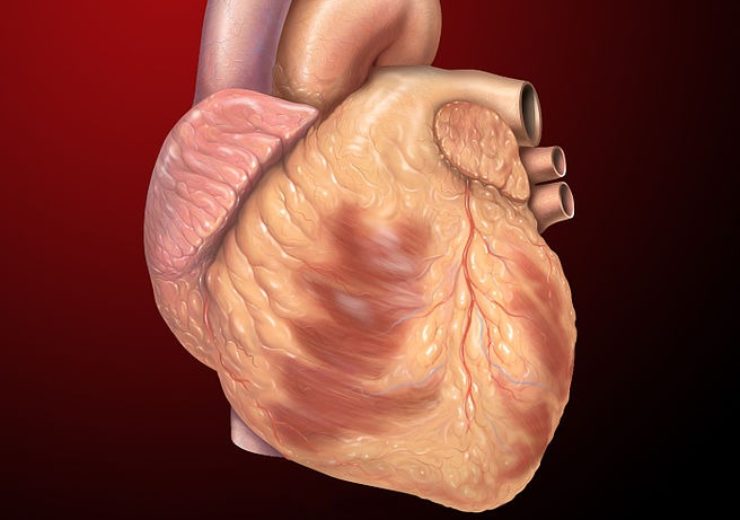
Image: Heart normal anterior exterior anatomy. Photo: Courtesy of Patrick J. Lynch, medical illustrator/Wikipedia.
CARMAT announced that the US Food and Drug Administration (FDA) has conditionally approved its Investigational Device Exemption (IDE) application for the Early Feasibility Study (EFS) of its total artificial heart in the US.
The company is engaged in development of an advanced total artificial heart to provide therapeutic alternative for people suffering from end-stage biventricular heart failure.
The medical device maker said that its EFS protocolconsists of 5 transplant-eligible subjects and is limited to a network of 7 institutions in US.CARMAT chief executive officer Stéphane Piat said: “The conditional approval to start a US study marks a significant milestone for CARMAT and the mechanical circulatory support field in general.
“This approval demonstrates the confidence of the FDA in our ability to conduct this feasibility study and reflects the high need for a safe and efficient solution for patients suffering from biventricular heart failure while waiting for a donor heart. We have already selected potential study sites and will immediately begin the submission process with the IRBs and research contract offices.”
Upon approval of IDE application with conditions, FDA provides a decision letter with number of subjects and investigational sites for the study, and allows the sponsor to start the subject enrolment.
CARMAT is expected to submit the clinical study documents to the Institutional Review Boards (IRB) of the selected study sites and is planning to start the patient enrolment for the study, upon obtaining the first IRB approval.
CARMAT’s total artificial heart resembles natural heart in its size
The company said that it has designed the total artificial heart to resemble the natural heart in its size, nature of structural materials and physiological functions.
The European Commission has granted a total of €33m (£29m) funding to CARMAT to develop the artificial heart, and the funding marks the largest subsidy ever given to an SME by Bpifrance.
In addition, the device, which avoids risk of rejection, would benefit thousands of patients annually and provide good quality of life, once the necessary clinical trials are completed.
