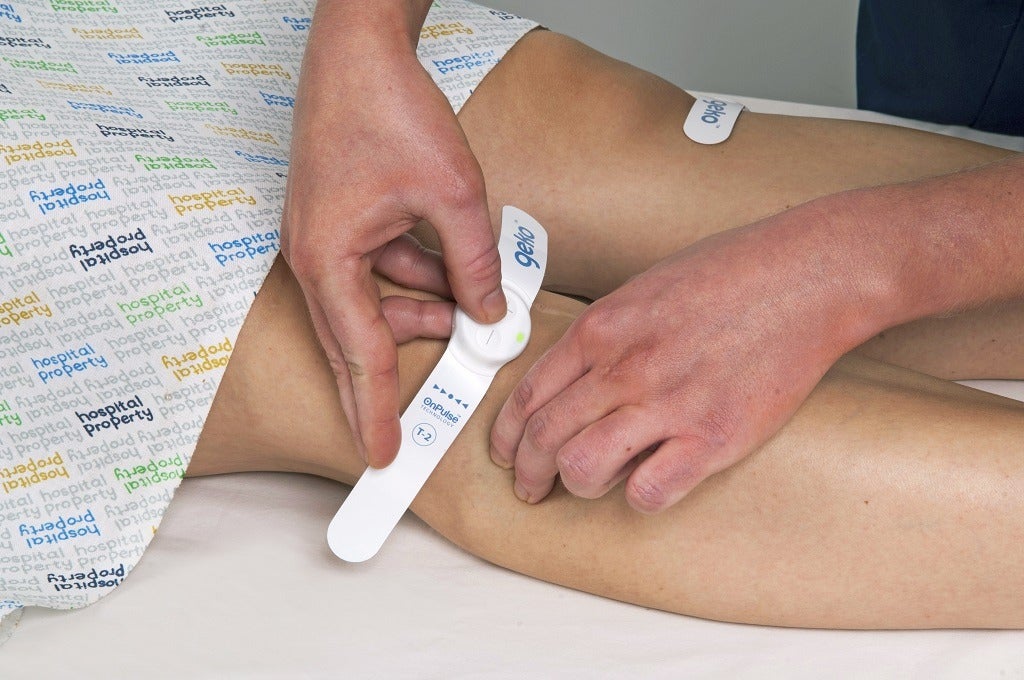
The geko device, a wearable, watch-sized device which attaches to the leg, uses electricity to stimulate nerves, creating blood flow equal to 60% of walking without the wearer having to move. In this article, Sky Medical Technology chief commercial officer Andrew Thelwell outlines Sky Medical’s steps for bringing the medical device to market, and highlights the considerable challenges of ‘crossing the chasm’ between the early adopters of the technology and the majority in the medical technology space.
Geoffrey Moore’s seminal book, Crossing the Chasm, has been a go-to source for technology entrepreneurs looking to take a product to the mass market.
Moore argues technology products do not follow a gentle path from early adopters to the majority but must cross a chasm between the two.
One of the universal truths I have experienced during my 25-year career has been the chasm is challenging to cross – never has that been truer than in bringing a medical device to market.
Opportunities in medtech
Innovation in medical technology is uniquely important to the future of healthcare. As populations age, we will need breakthrough medical technology to bridge the gap between delivering an affordable healthcare system and maintaining high levels of care that patients expect.
Particularly important are ‘platform’ innovations: those technologies that solve more than one medical issue.
OnPulse is one such technology – used to stimulate the common peroneal nerve and activate calf and foot muscle pumps, resulting in muscle contraction in the lower leg.
This has around 60% of the impact on blood flow increase in the deep veins as walking would have, but without raising someone’s pulse or blood pressure.
We spent time turning this technology into a medical device, where that capability to move blood around a person could be brought to life.
By 2015 we had incorporated OnPulse technology into the geko device, that could offer positive patient outcomes to a number of medical conditions.

Market potential
The first critical part of the journey to commercialisation was to focus on the most important potential markets for the device.
We were able to focus on three key medical issues: the prevention of venous thrombosis (VTEs); the prevention and treatment of oedema (swelling); and wound therapy applications (hard-to-heal leg ulcers).
The total addressable market opportunity for the geko device in each of these areas is £202 million, £282 million, and £603 million respectively in 2020.
Creating this focus did not come without its challenges. Investors, board members and employees all had their own thoughts on application areas, and it was important to demonstrate these three application areas offered the best route to commercial success, based not only on the size of market but also how our product stacked up against other forms of treatment for these medical conditions.
Regulatory challenges
Clearly one of the ways in which medical products differ from other technical products is they need to meet regulatory requirements of different health services around the world.
Building positive patient evidence is a critical part of the process of gaining regulatory approval.
Sky Medical was able to receive National Institute for Health and Care Excellence (NICE) guidance based largely on an early prototype and the OnPulse technology’s performance in early evaluations.
The work of Dr Natarajan in Stoke helped the geko device gain US Food & Drug Administration (FDA) clearance for blood clot prevention.
Despite being the first bioelectronic muscle pump activator of its kind to be cleared by the FDA for VTE prevention across all patients, including non-surgical patients, this and NICE guidance still does not guarantee global market access.
One of the most important reasons to continue collecting data, as adoption grows, is to satisfy the varied requirements of healthcare systems globally. Each wave of new positive patient data provides fresh evidence to support the product’s extended reach into new geographies and medical use applications, and the breadth of permitted claims directly arises from what the data has been able to demonstrate.
The economics realities
Healthcare systems base decisions on multiple factors – for example the investment required and payback period.
There may be a clear overall financial benefit, but the cost of adoption arises before the financial upside, so it may be difficult to realise within annualised budgets.
Equally, the cost may appear in one budget, but the benefit may accrue across a number of other budgets.
Even when the economic case is unarguable from all of these perspectives, other issues can limit the speed of adoption; in one case we were slowed by a hospital that had just reordered prescription chart stationery for an entire year which would need changing to incorporate our product.
It is essential companies recognise multiple stakeholders are involved in the decision making, all with their own responsibilities and accountabilities.
Companies can help speed adoption by providing support for aspects such as staff training, information manuals, product videos as well as better understanding the process through which innovation is adopted in healthcare services and being sympathetic to it.
Always remember it is not the primary focus of a clinician to see that your product is adopted, so providing the appropriate support is essential.
Bridging the chasm is tough in any technology business but in medical technology the bar to bringing innovation to the mass market is rightly extremely high.
Companies need to show patience. Technology investors – perhaps more used to a five-to-seven-year runway before seeing a return on investment – need to remain patient and see that, while the timescales may be longer in medical technology, the rewards can also be more significant.
Changing times
All of this means the chasm between early adopters and the early majority in medical technology can seem more like a canyon; medical innovation requires a high level of evidence to ensure technology that does get adopted has been through the most rigorous processes.
Technology adoption relies on the commitment, expertise and determination of clinicians, who carry the torch of innovation through the system, driven only by the passion to improve patient outcomes.
There is now growing evidence that things are changing. The medical challenges of Covid-19 have forced faster evaluation of new medical innovation with some medical stakeholders reporting as much innovation adoption has taken place in the past 12 months as in the entire decade previously.
The launch of the MedTech Funding Mandate in the UK on 1st April 2021 will further support devices, diagnostics or digital products that are:
- demonstrably effective, proven through positive NICE guidance
- delivering material savings to the NHS and cost saving within the year
- affordable to the NHS
The implementation of this mandate should help accelerate the deployment of proven, safe and cost-effective medical technology in the NHS, reducing the burden on clinicians to be the champions for innovation.
It is hoped this mandate will equip hospitals and other providers with the necessary financial support to fund the initial pain of new innovation adoption and realise the mid-to-longer-term benefits more consistently.
A journey worth taking
Medical technology is critical to bridging the gap between today’s healthcare needs and tomorrow’s.
Despite this, the bar for mass global adoption remains – rightly – extremely high.
To cross the chasm – or canyon – between the early adopter and the early majority requires a combination of a proven technology that is safe and effective, global regulatory approval driven by evidence based positive patient outcomes, a cast iron and nationally tailored economic case, alongside a clear understanding of the processes through which innovation adoption takes place.
It will still likely take a considerable amount of time to execute successfully, but for those like Sky Medical that do so, the economic and societal benefits make the journey fully worthwhile.






