Vercise Genus system is used to treat symptoms of Parkinson's disease, essential tremor, and dystonia by offering accurately targeted electrical stimulation in the brain
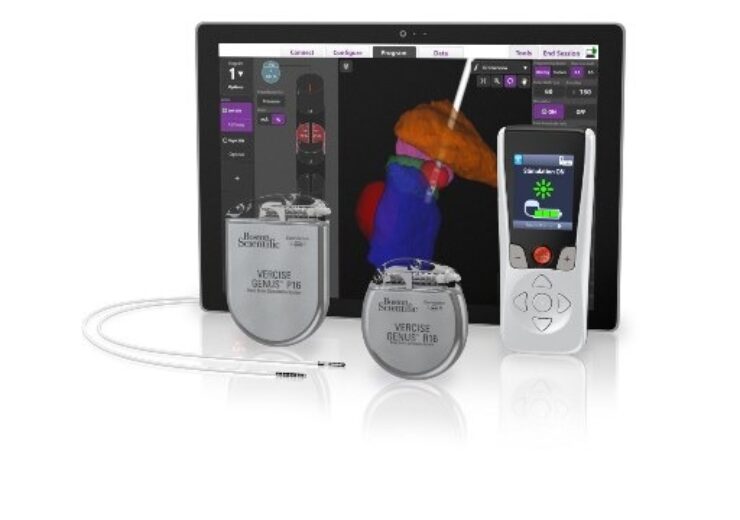
Boston Scientific has secured CE mark for fourth generation Vercise Genus DBS system. (Credit: Boston Scientific Corporation)
Boston Scientific has secured CE mark approval for its fourth-generation Vercise Genus deep brain stimulation (DBS) system.
With full-body MRI conditional and Bluetooth capabilities, the Vercise Genus system is used to treat symptoms of Parkinson’s disease (PD), essential tremor, and dystonia by providing accurately targeted electrical stimulation in the brain for optimal symptom relief.
Vercise Genus offers various benefits to patients, including a low-profile two-in-one extension with the option of abdominal placement.
New clinician software enhances programming with integrated visualisation using patient imaging through the firm’s exclusive relationship with Brainlab.
Vercise Genus, which is the fourth-generation DBS system since 2012, is based on innovations such as multiple independent current control (MICC) and the Cartesia directional lead with integrated visualisation.
Boston Scientific has begun a limited market release of the new Vercise Genus DBS system
The company has also commenced a limited market release of the fourth-generation Vercise Genus DBS system in Europe.
In Europe, the Vercise Genus DBS system is designated for use in unilateral or bilateral stimulation of the subthalamic nucleus or internal globus pallidus to treat levodopa-responsive PD, which is not abundantly controlled with medication.
It is also indicated to treat intractable primary and secondary Dystonia for persons seven years of age and older.
In addition, Vercise Genus DBS system with thalamic stimulation enables to suppress tremor that is not abundantly controlled by medications in patients diagnosed with essential tremor or PD. The Vercise Genus DBS system is not yet commercialised in the US.
Boston Scientific neuromodulation president and senior vice president Maulik Nanavaty said: “For patients, the Vercise Genus DBS System continues the tradition of small, thin devices, and it provides Bluetooth programming which is important during times of social distancing.
“Combined with the option of a 25-year rechargeable battery as well as the expanded MRI conditional1 feature available on our primary cell devices, patients can find the best option to suit their specific needs.”
In June this year, Boston Scientific secured 510(k) clearance from the US Food and Drug Administration (FDA) for its LUX-Dx insertable cardiac monitor (ICM) system.
