BD Veritor Plus System is designed for serial testing in any setting with a CLIA certificate of waiver
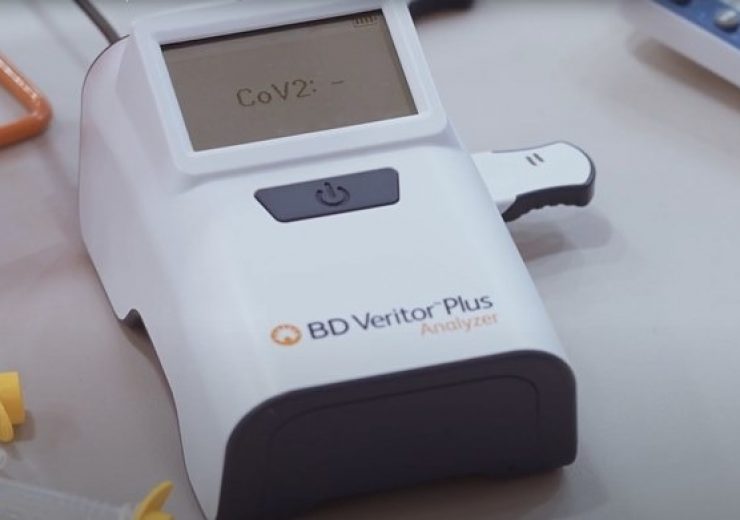
BD Veritor Plus System suitable for use in everyday locations such as schools and businesses. (Credit: BD)
Becton, Dickinson and Company (BD) has secured an emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for its rapid antigen test to screen SARS-CoV-2 via serial testing of asymptomatic individuals.
The BD Veritor Plus System for Rapid Detection of SARS-CoV-2 will facilitate the qualitative detection of SARS-CoV-2 nucleocapsid antigens in direct anterior nasal swabs from individuals who are either suspected of Covid-19 by their health care provider within the first five days of the onset of symptoms.
It will help in the qualitative detection of SARS-CoV-2 nucleocapsid antigens from individuals without symptoms or other epidemiological reasons to suspect Covid-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests
The BD Veritor Plus System is designed for serial testing in any setting with a CLIA certificate of waiver.
The system is suitable for use in everyday locations such as schools and businesses, in addition to serial testing in other situations such as athletes and teams to ensure safe games and competitions.
According to the company, the RADx-funded study shown that the serial use of diagnostic tests, including rapid antigen tests, increased the ability to identify infection.
BD life sciences president Dave Hickey said: “Frequent testing of individuals without symptoms will enable those with negative results to resume their normal school or work routines and will help to identify and isolate positive cases of Covid-19 as early as possible to prevent further spread.
“Screening through serial testing is an important part of any back-to-school or back-to-work program, along with additional measures such as mask wearing and social distancing.”
In February this year, BD collaborated with smartphone-enabled at-home medical tests provider Scanwell Health to develop an at-home rapid test for Covid-19.
