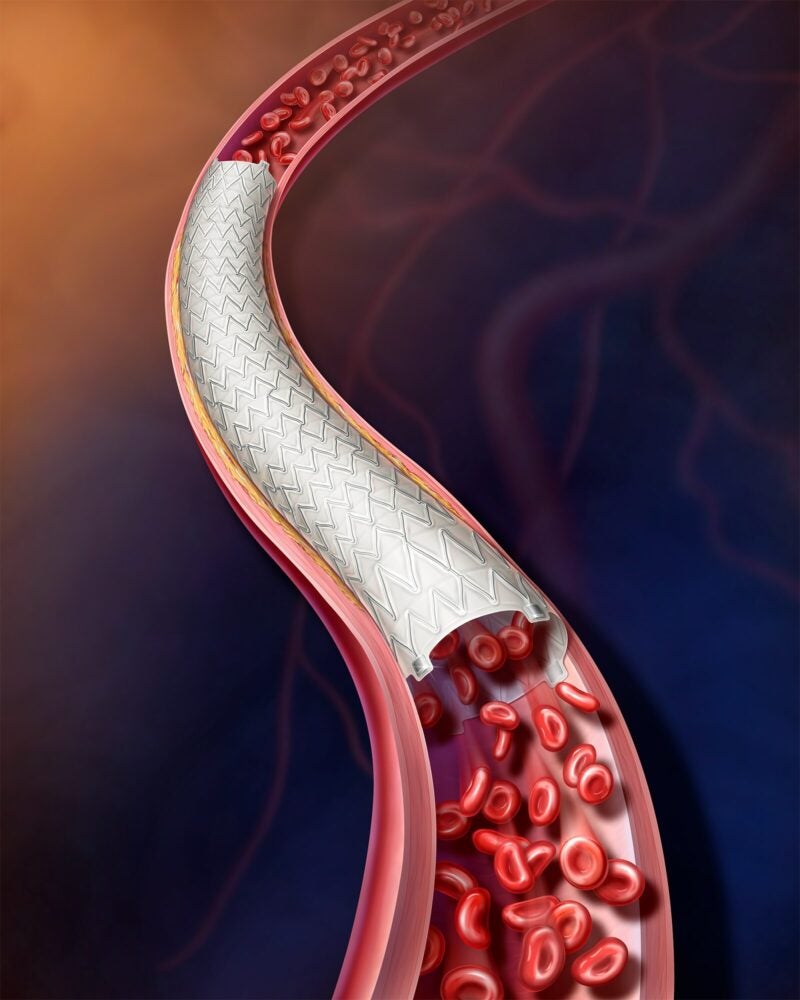
BD (Becton, Dickinson and Company) has enrolled the first patient in an investigational device exemption (IDE) AGILITY study of its BD Vascular Covered Stent.
The Vascular Covered Stent, which is under investigation, is a low-profile, self-expanding nitinol implant enclosed in polytetrafluoroethylene.
It is administered using a delivery mechanism that allows for regulated stent release.
The global, prospective, multi-centre, single-arm, non-randomised AGILITY intends to study the safety and effectiveness of the vascular-covered stent in the treatment of peripheral arterial disease (PAD).
The trial will recruit 315 patients at up to 40 clinical study sites across the US, Europe, Australia and New Zealand.
Every treated patient will have follow-up at different intervals following therapy, ranging from one month to three years.
BD Peripheral Intervention vice president and general manager Tim Hug said: “There continues to be significant unmet needs in the treatment of PAD patients.
“We are excited to have initiated this study and evaluate the treatment benefits of this potentially differentiated technology.
“This stent could give interventionalists an important new solution in the fight against PAD, expand our portfolio and enable us to better serve our customers and the patients they treat.”
The first patient in the trial was enrolled at Trinity Medical Center in Bettendorf, Lowa, US.
BD said that minimally invasive techniques using drug-coated balloons, angioplasty balloons, atherectomy and covered stents can be used to increase blood flow through the diseased areas.
AGILITY study national principal investigator Sean Lyden said: “When we’re addressing advanced PAD, a self-expanding covered stent can play an important role.
“We need a stent that can track to the lesion, apposes the vessel wall and ultimately provides long-term durability. We’re excited to see how this technology performs.”
In January this year, the medical device firm signed a collaboration agreement with US-based Techcyte to develop an artificial intelligence (AI)-based digital cervical cytology system for Pap testing.




