The solution includes a combination of 4-6 channel small foot-print real-time PCR instruments called the Maverick qPCR and one-step multiplex RT-PCR reagent in lyophilised format
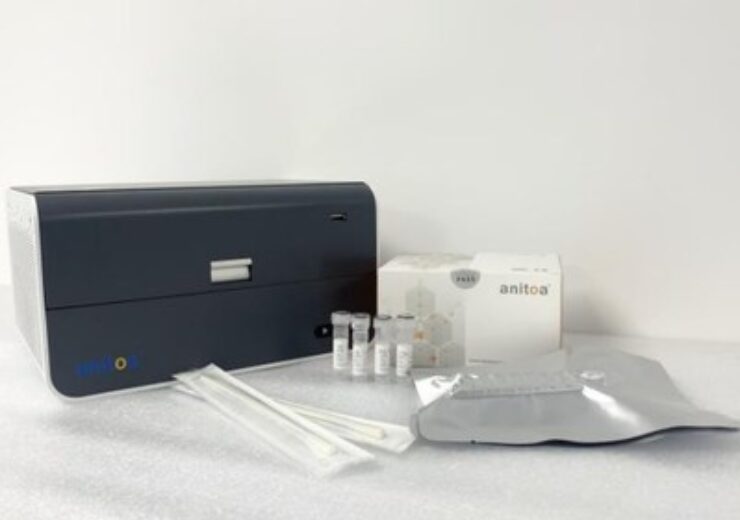
Anitoa’s RT-PCR solution includes a family of 4-6 channel small foot-print real time PCR instruments and a one-step multiplex RT-PCR reagent. (Credit: Anitoa Systems, LLC)
US-based medical device technology firm Anitoa Systems has launched a new portable RT-PCR Molecular assay to detect the dengue virus.
The solution includes a combination of 4-6 channel small foot-print real-time PCR instruments known as the Maverick qPCR and one-step multiplex RT-PCR reagent in lyophilised format.
According to Anitoa, the Maverick real-time PCR devices are lab-accurate multi-plex qPCR equipment in a tiny format. They are battery operated (including car batteries) and consume very little table space.
Anitoa used its patented CMOS biosensor technology in Maverick qPCR devices.
Maverick qPCR devices are used for on-site testing of food, environmental samples and agriculture products due to their durable all-solid-state construction, compact form factor, and low cost.
Anitoa Systems chief executive officer Zhimin ding said: “We are excited to be part of the community that offers advanced diagnostic solutions for a series of neglected tropical infections.
“We would like to use our technology and expertise to improve our ability to address this unmet market need.”
The firm’s multiplex test detects several target genes in a sample, which are considered to be highly expressed in patients that later develop serious symptoms.
Alternative antibody-based diagnostics are less reliable and predictive than nucleic acid testing such as multiplex RT-PCR. Early diagnosis and management can lead to a better outcome for patients and relieve the costs on families and health care institutions, Anitoa said.
The new dengue virus detection solution is part of Anitoa’s ongoing programme to develop tests to aid in the fight against several neglected tropical illnesses in low-resource settings.
In January this year, Anitoa Systems expanded its SARS-CoV2 rapid nucleic acid test portfolio with the addition of two new kits to detect Omicron and Delta mutations.
Anitoa is a medical device technology start-up established in 2013, with an aim to offer integrated and portable biosensor devices for molecular detection, based on its unique CMOS image sensor-based fluorescence and chemiluminescence molecular imaging technology.
