The company designed AccuCinch System to provide a minimally invasive transcatheter therapy for patients living with symptomatic heart failure (HF) with reduced ejection fraction (HFrEF)
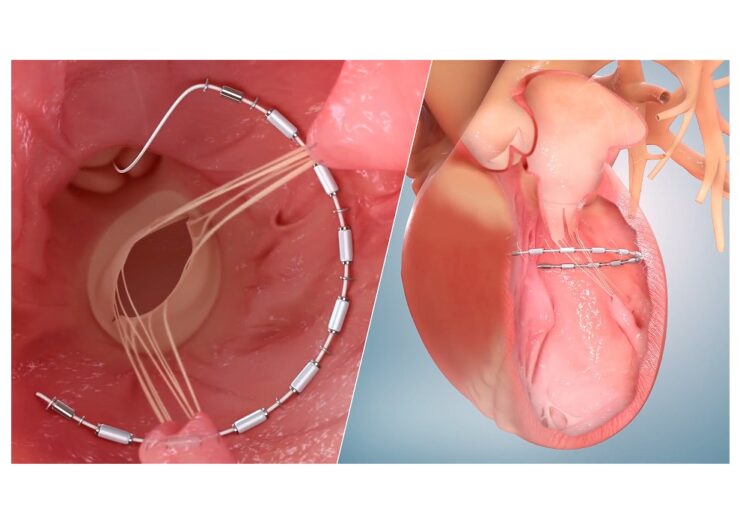
Ancora Heart’s AccuCinch ventricular restoration system. (Credit: Business Wire/Ancora Heart, Inc.)
US-based medical device maker Ancora Heart has received the US Food and Drug Administration (FDA) Breakthrough Device Designation for its AccuCinch ventricular restoration system.
The AccuCinch system is designed to offer a minimally invasive treatment option for patients living with symptomatic heart failure (HF) with reduced ejection fraction (HFrEF).
It is an advanced device-based therapy system that provides a new treatment option for HF patients, whose disease progressed beyond the ability of medications and pacemakers.
The AccuCinch System bridges the gap between medication or pacemaker therapy and left ventricular assist devices (LVADs) or a heart transplant, said Ancora.
The investigational device is currently being evaluated in the CORCINCH-HF clinical trial.
Ancora Heart president and CEO Jeff Closs said: “Ancora Heart is dedicated to developing solutions that address the unmet need for improved treatment options for heart failure patients.
“The Breakthrough Designation is a valuable step in the pathway to FDA approval and we look forward to continuing to work closely with the agency in order to make the AccuCinch System available to these patients.”
The transcatheter procedure using AccuCinch System involves attaching a flexible implant to the inner wall of the left ventricle, which is then cinched to reduce the size of the left ventricle.
The implant is intended to reduce stress on the ventricular wall, along with supporting and strengthening the heart wall.
Ancora Heart claims that its AccuCinch System is the only completely transcatheter device designed to restore the structure and function of the enlarged left ventricle of the heart.
The ongoing CORCINCH-HF trial is evaluating the safety and efficacy of the AccuCinch ventricular restoration system in HFrEF patients.
The study is said to support the submission of its Premarket Approval (PMA) application to the US Food and Drug Administration, said the company.
CORCINCH-HF study global co-principal investigator Ulrich P Jorde said: “The chronic and progressive nature of heart failure leaves many patients to face an increasingly challenging future as existing treatments, including medications and pacemaker devices, are not able to keep the disease at bay.
“Early data on the AccuCinch System are promising, providing hope that this novel transcatheter approach may address the therapeutic gap for heart failure patients in the future.”
