US-based medical device technology company Abiomed has received the US Food and Drug Administration (FDA) pre-market approval (PMA) for Impella RP Flex with SmartAssist.
The PMA, which is the US FDA’s highest level of approval, allows the use of Impella RP Flex with SmartAssist as safe and effective to treat acute right heart failure for up to 14 days.
Impella RP Flex is indicated for patients with acute right heart failure following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery.
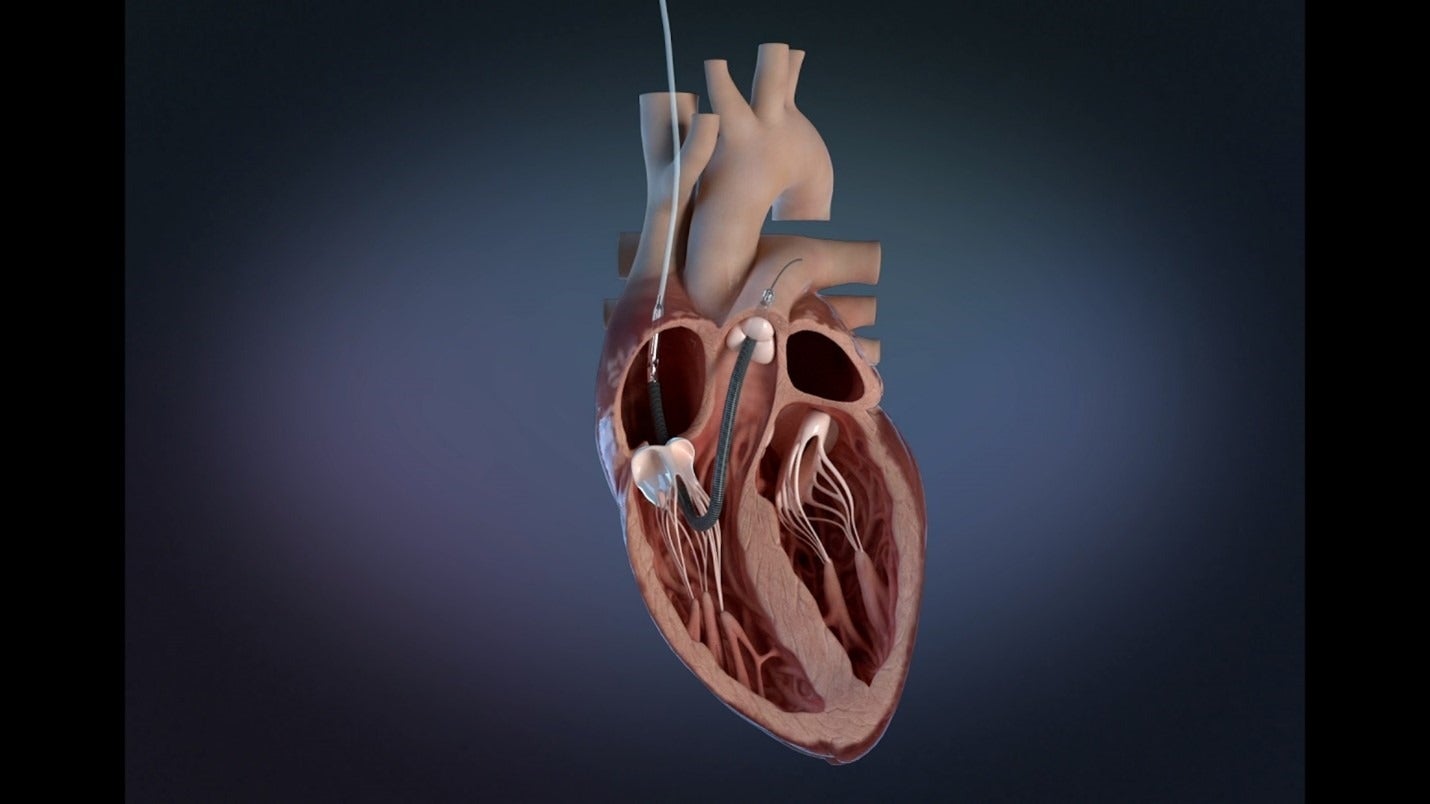
Implanted through the internal jugular (IJ) vein, the device features SmartAssist dual-sensor technology to optimise patient management and enables patient mobility.
Also, it features the world’s smallest percutaneous right heart mechanical circulatory support (MCS) technologies to help patients achieve native heart recovery.
They do not require extracorporeal blood circulation and remain the only MCS technologies with FDA PMA indications for the treatment of right heart failure.
Hackensack Meridian Health Heart and Vascular Hospital cardiothoracic surgeon and department of cardiac surgery chairman Mark B Anderson said: “Impella RP Flex demonstrates Abiomed’s ongoing commitment to improving patient survival and achieving native heart recovery.”
Kingwood Medical Centre interventional cardiologist and cardiovascular research director Robert Salazar said “The complexity of right ventricular failure has resulted in patients being underdiagnosed and undertreated.
“Impella RP Flex is a novel tool that gives physicians the flexibility to treat this challenging patient population.”
Abiomed said that Impella RP Flex offers single venous access through the IJ vein, and 11 French (Fr) indwelling catheters to facilitate patient mobility.
It features a flexible cannula over an extra-support guidewire to ease the insertion and pump delivery, and heparin-free purge to simplify the patient’s anticoagulant management.
The SmartAssist dual-sensor technology with Impella Connect will provide advanced metrics to help with pump management and weaning, said the company.
Furthermore, Abiomed intends to start commercialising the Impella RP Flex in the US through a controlled rollout this quarter.