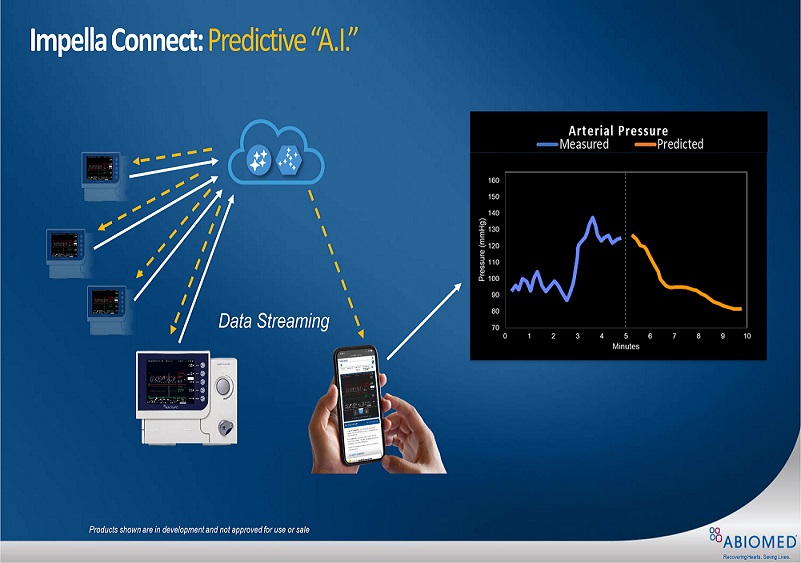
The FDA approval allows streaming of console data through Impella Connect to a secure server, where AI could offer predictive clinical information
Abiomed is providing the data streaming capability through the Impella Connect interface. (Credit: Business Wire)
Abiomed has secured approval from the US Food and Drug Administration (FDA) for one-way digital data streaming during patient support from Automated Impella Controller (AIC).
AIC is the external console used with Impella heart pumps.
The company’s FDA approved Impella heart pumps are used to treat heart attack or cardiomyopathy patients in cardiogenic shock. The heart pumps include Impella 2.5, Impella CP, Impella CP with SmartAssist, Impella 5.0, Impella LD, and Impella 5.5 with SmartAssist.
Abiomed is providing the data streaming capability through the Impella Connect interface.
Currently installed at more than 200 hospitals, Impella Connect interface is a cloud-based remote monitoring platform.
Abiomed develops AI algorithm to predict patient’s arterial pressure
The FDA approval allows streaming of console data through Impella Connect to a secure server, where artificial intelligence (AI) technology could offer predictive clinical information to the patient’s physician.
Based only on the prior five minutes of console data, an AI algorithm already trained by Abiomed will be able to predict the next five minutes of a patient’s arterial pressure.
The company has also developed AI algorithms to predict other parameters, such as stroke volume, left ventricular pressure and cardiac output.
However, the AI algorithms are not yet to be cleared or approved for patient use.
Abiomed intends to submit them for regulatory review, once they are fully developed.
Abiomed’s chief medical officer Chuck Simonton said: “Artificial intelligence networks, properly trained using large volumes of streaming data, can be powerful tools to aid in clinical decision-making.
“One day, using artificial intelligence, physicians may be able to confidently predict a patient’s future hemodynamics. That would make clinical decision-making more efficient and improve patient outcomes.”
In June, Abiomed received the emergency use authorization (EUA) from the FDA for Impella RP to treat patients suffering from Covid-19 related right heart failure or decompensation, including pulmonary embolism (PE).
