The FreeStyle Libre 2 is said to be the only iCGM system with optional real-time alarms, which measures glucose levels every minute
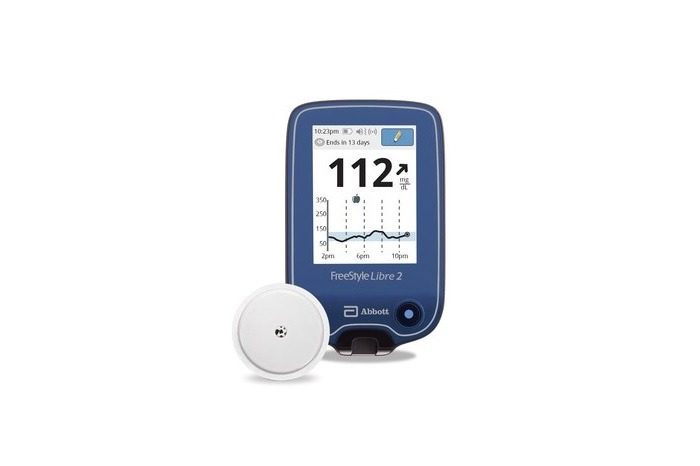
Abbott’s FreeStyle Libre 2 sensor. (Credit: Abbott)
Abbott has secured approval from the US Food and Drug Administration (FDA) for its next-generation FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system for adults and children aged between four and older with diabetes.
The FreeStyle Libre 2 is claimed to be the only iCGM system with optional real-time alarms, which measures glucose levels every minute. The self-applied iCGM sensor helps to eliminate the risk of fingersticks.
The system uses Bluetooth technology to automatically alert users when their glucose is high or low without requiring to scan the sensor. The users are also provided with an option of turning off the customisable and real-time alarms.
Abbott’s FreeStyle Libre 2 system can digitally connect and communicate with other devices
Abbott’s FreeStyle Libre 2 system has the capacity to digitally connect and communicate with other devices, enabling patients to better manage their diabetes.
The FreeStyle Libre 2 next-generation sensor has to be worn on the back of the upper arm for up to 14 days for measuring glucose every minute, helping users and their healthcare providers to take better treatment decisions.
The users can view their glucose reading, trend arrow and eight-hour history with a one-second scan using a handheld reader.
Abbott will sell the new system at participating pharmacies and durable medical equipment suppliers (DMEs) across the US.
Abbott diabetes care senior vice president said: “We’re thrilled to bring our next generation technology on our world-leading sensing platform to both children and adults with diabetes in the US.
“With unsurpassed 14-day accuracy and enhanced features including optional alarms at a fraction of the cost of other CGMs, Abbott’s FreeStyle Libre 2 system will change the future of diabetes care in the US the same way it has around the globe.”
In May, Abbott secured emergency use authorisation (EUA) from the FDA for its SARS-CoV-2 IgG lab-based serology blood test on the Alinity i system.
