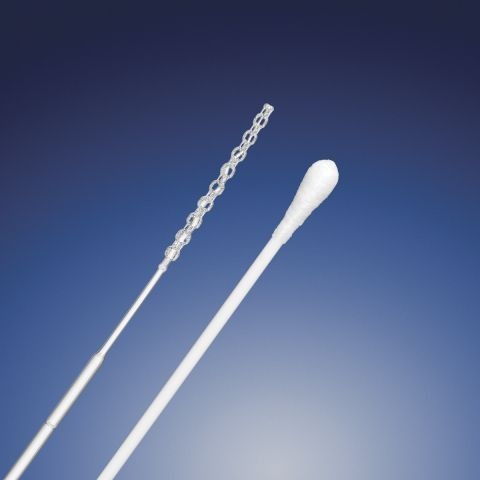
A new 3D-printed nasal swab offers a “cost-effective” alternative to traditional models used in Covid-19 testing.
It was developed by US in vitro diagnostic manufacturer Resolution Medical – which has partnered with fellow American firm and medical device component supplier Qosina to release the product.
The new sterile, nasopharyngeal swab can be rapidly produced using industry-approved materials and Digital Light SynthesisTM (DLSTM) – an innovative 3D printing process provided by California-based digital manufacturing firm Carbon 3D.
The 5.9 inch-long swab comes with a ‘flocked’ design, meaning its surface features multi-length fibres for improved sample collection, and Qosina claims its lattice-structured dome tip offers comfort and durability to optimise the patient experience.
Scott Herskovitz, president and CEO of Qosina, said: “As a leader in swabs provided to the medical industry, we are excited to add the 3D-printed swab to our product offering.
“Its creative design meets the growing demand from our customers for high-quality Covid-19 testing swabs.
“Our mission is to provide the best customer experience possible by helping our customers find, order and receive quality medical components quickly and accurately, and this partnership is the perfect fit to support our mission.”
The need for 3D-printed nasal swabs
At the beginning of October, Qosina claimed that increased production of 3D-printed swabs “may be the best answer” to meet the ever-growing need for flocked swabs – the specific type of long, thin swabs needed to take SARS-CoV-2 samples from the upper throat via the nose.
The two global companies that manufacture these swabs – Italian firm Copan Diagnostics and US-based medical supplier Puritan – have reportedly been overwhelmed by the global demand for their products.
According to data from the volunteer-run COVID Tracking Project, there are currently close to one million new Covid-19 tests being conducted in the US each day – with this number likely to increase over the coming weeks and months.
Qosina itself stated that some sources believe the number of weekly diagnostic coronavirus tests could rise to between 20 and 30 million over the course of the next 18 months – but added 3D printing’s ability to produce high volumes of small, complex products could help the US meet these demands.
The company also claimed that the benefits of 3D-printed swabs will extend beyond the pandemic, and their popularity will rise due to their “superior quality” compared to traditional swabs, as well as the fact they cause patients less discomfort during application and are more efficient because they can be printed on demand.



