All articles by Vidya Sagar
Fluidx Medical’s Embolic Device shows novel visibility in in-vivo study
Fluidx Medical Technology, the developer of advanced embolic material GPX, has announced positive visibility results for its GPX-Clear Embolic Device…
Paragonix LUNGguard enables safe transport of donor lungs across record distance
Paragonix Technologies announced that its Paragonix LUNGguard System has achieved a milestone of safely completing a donor lung transport from…
ZEPHYRx and MIR Join Forces to Market Remote Respiratory Monitoring Solutions
ZEPHYRx, LLC and MIR, Medical International Research, today announced they have entered into an exclusive partnership to jointly market the ZEPHYRx Remote Respiratory…
CytoSorbents enrolls first patient in PROCYSS trial of CytoSorb
US-based critical care company CytoSorbents has enrolled first patients in the PROCYSS trial evaluating CytoSorb to reverse refractory septic shock…
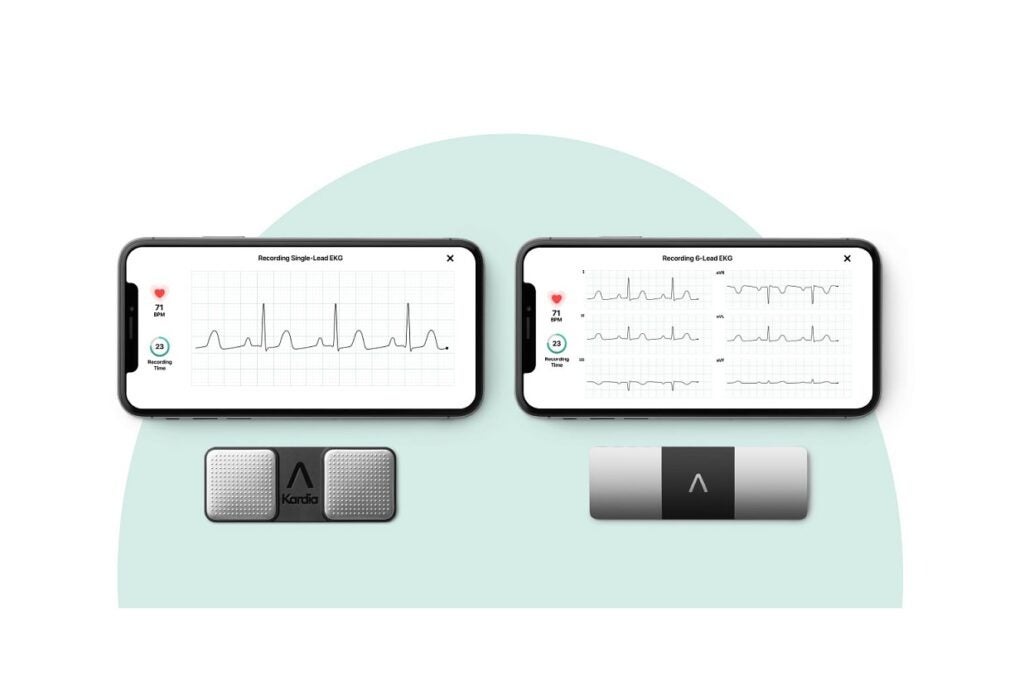
AliveCor gets FDA nod for credit-card-sized personal ECG device
AliveCor has received the US Food and Drug Administration (FDA) approval for KardiaMobile Card, a personal ECG device designed to…
ZAP Surgical Announces Installation of its Pioneering Radiosurgery Platform at Japanese Bullet Train Station
ZAP Surgical Systems, Inc. today announced installation of its first ZAP-X® Gyroscopic Radiosurgery® platform in Japan. Using a new vault-free…
Vynca raises $30m funding to expand palliative care platform
Vynca, a technology-enabled healthcare provider for serious illnesses, raised $30m funding to expand its integrated palliative care platform. Led by…
Alex Therapeutics, Pfizer partner to advance evidence-based digital therapeutics in Germany
Alex Therapeutics and Pfizer have joined forces to provide evidence-based, clinically validated and personalised digital therapies to patients. Under the…
GI Dynamics Announces Approval of the I-STEP Clinical Study in India
GI Dynamics Inc., a medical device company that is developing the EndoBarrier® System for patients diagnosed with type 2 diabetes…
FDA accepts label updates to Suneva’s Plasma IQ device
Suneva Medical announced that the US Food and Drug Administration (FDA) has accepted the updates to the Plasma IQ label,…