All articles by
Penumbra announces FDA clearance of Indigo aspiration system for treatment of pulmonary embolism
Penumbra, a global healthcare company focused on innovative therapies, today announced U.S. Food and Drug Administration 510(k) clearance for expanded…
Medirom announces partnership with MATRIX Industries to deliver world’s first health monitoring wearable that never requires charging
Medirom is announcing a partnership with MATRIX Industries enabling users in the US and Japan to monitor their health activities…
KDx Diagnostics gets FDA breakthrough device status for URO17 bladder cancer recurrence test
KDx Diagnostics has secured breakthrough device designation from the US Food and Drug Administration (FDA) for its URO17 urine test…
Medtronic buys DTM spinal cord stimulation therapy provider Stimgenics
Medical technology firm Medtronic has acquired US privately-held company Stimgenics for an undisclosed sum. Based in Bloomington of Illinois, Stimgenics…
Phillips-Medisize expands clinical manufacturing capabilities to serve growing drug delivery device market
Phillips-Medisize, a Molex company, announced expansion of its current Global Innovation and Development (GID) site in Struer, Denmark with a…
3Derm announces two FDA breakthrough device designations for autonomous skin cancer AI
3Derm Systems, a leader in the skin imaging and diagnostics industry, announced today that it has been granted two FDA…
Sebia enters into agreement with Sanofi to develop multiple myeloma diagnostic test
Sebia (Lisses, France), a world leader in multiple myeloma diagnostics and monitoring, announces today that it is entering into an…
Mobidiag secures additional funding from Business Finland for rapid sepsis diagnostic test
Mobidiag, a revenue generating, molecular diagnostics company with complementary platforms that address antimicrobial resistance and other areas of unmet diagnostic…
BioMarker Strategies awarded Phase II National Cancer Institute Contract to develop novel predictive test
BioMarker Strategies announced that the National Cancer Institute (NCI) has awarded the company a Phase II Small Business Innovation Research…
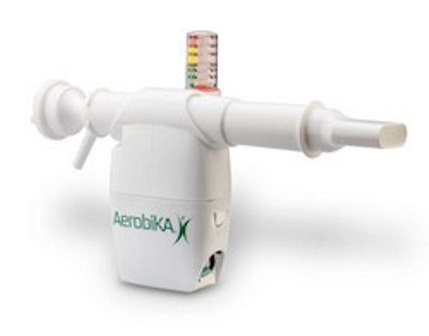
FDA approves Monaghan Medical’s tandem pulmonary therapy device
Monaghan Medical (MMC) has secured approval from the US Food and Drug Administration (FDA) for a new respiratory treatment option.…