It is no surprise that the global orthopaedic devices market is prolifically expanding, and the reason is an ostensible rise in the number of orthopaedic surgery procedures being performed worldwide.
Reportedly, in 2017, approximately 22.3 million orthopaedic surgery procedures were performed globally.
This dramatic surge in orthopaedic procedures has revealed a significant need for orthopaedic devices.
Globally, the orthopaedic devices market is poised to record an annual remuneration of $60bn by 2027.
Orthopaedic devices play an indispensable role in offering pain relief, enhancing the quality, and improving the mobility of patients suffering from musculoskeletal disorders and anomalies.
These medical equipment and procedures used in orthopaedics keep on transforming almost every day considering the changing consumer needs.
Technological advancements and integration of digital technologies currently serve as the predominant driver for upscaling the proceeds of the orthopaedic devices market.
How will the global orthopaedic devices market respond to the ongoing technological developments?
Maintaining a qualified and well-experienced team of professionals is becoming increasingly essential for the healthcare companies subject to rapid technological advancements.
It is quite important for the developers and manufacturers to keep in mind the comfort, safety, and convenience of the users while designing prototype medical equipment.
That said, the incorporation of cutting-edge trends has indeed revolutionised the field of orthopaedics over the last few years – for example the introduction of wearable medical devices and medical fabrics.
Reshaping the future of orthopaedic healthcare with wearable technology
Wearable technology stands out to be an exciting venture which has gained massive traction around the globe over the past few years.
This technology enables various industrial professionals and individuals to track personal fitness and health parameters and is now becoming more and more precise with each contemporary advancement.
As these devices continue to expand in accuracy and gain further utilities in health monitoring, their potential to influence orthopaedic care is anticipated to experience manifold growth in the near future.
Orthopaedic specialists may use this technology to analyse and evaluate the pre-operative course of their patients, who can communicate various factors related to care, without actually needing to be physically attended.
Various device developers have now been going nine yards for bringing novel innovations in the orthopaedic business space, postulating the devices’ future use in orthopaedic care.
In December 2020, Exactech, a joint replacement solutions developer, took over one of the finest patient wearable device producers, Muvr Labs, for an undisclosed deal.
The latter company’s portfolio is designed to support surgeons’ engagement with joint replacement recipients throughout the care process.
Moreover, Muvr’s platform encompasses mobile device applications, wearables, and chatbot texting to enable the remote monitoring of patients while healthcare teams receive real-time information for the patients’ recovery and experience.
In yet another instance, Pixee Medical, a France-based medical conglomerate, had announced completion of its first total knee replacement surgery directed by the Vuzix M400 AR smart glasses.
As per a news report provided by Vuzix Corporation, the surgeon’s performance using AR+ for a total knee replacement operation marks a revolutionary milestone in the advanced knee replacement surgery.
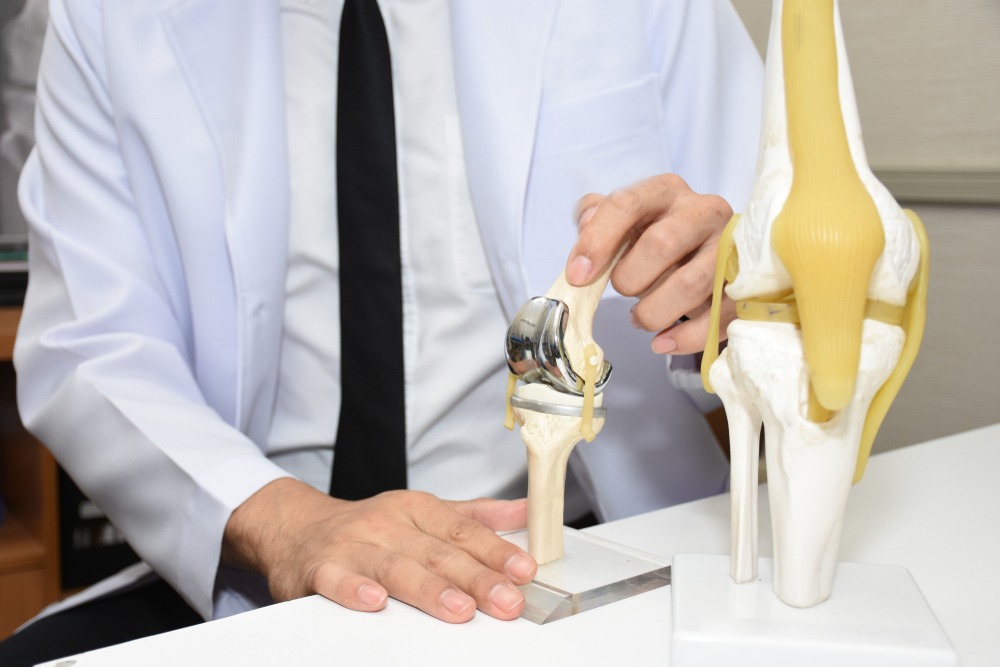
Knee replacement procedures
Here are some of the projected facts and figures associated with knee replacement surgeries and procedures:
- By 2030, total knee replacement surgeries are predicted to grow up to 3.5 million procedures per year.
- Almost half of American adults are likely to develop knee osteoarthritis in at least one knee in their lifetime.
- 80% of osteoarthritis patients have been diagnosed to have some degree of movement limitation.
These forecasts have bolstered the adoption and deployment of orthopaedic devices, and in this case, knee replacement devices.
Various companies in this business space have been making ground-breaking revelations and achieving incredible milestones via introducing novel product developments, and much more.
One such industrial conglomerate, Zimmer Biomet, had received an FDA nod for its ROSA partial knee system for robotically supported partial knee surgery.
The newly acclaimed system is one of the newest additions to ROSA Robotics and Zimmer Biomet’s diverse application robotics platform, which encompasses the ROSA Knee System for knee anthroplasty and ROSA ONE for spine and neurosurgical procedures.
‘Healing’ the growth curve of the orthopaedic devices industry in the US
According to the United States Department of Transportation, over six million motor accidents occur almost every year.
In these car crashes, knee injuries and spine injuries are an all too common occurrence.
Knee injuries cost an average of $50,000 for knee replacement procedures, while for spine injuries, the surgery could cost more than $150,000 in Florida, revealing an opportunity for cost-cutting orthopaedic devices in the state.
Medical therapies and procedures that reduce costs and time and offer optimised and personalised outcomes are likely to expand as innovative technologies continue to shape up the industry.
Intelligent orthopaedic devices- a blend of traditional techniques and high-end technology – are expected to be the future of the industry in 2021 and beyond.






