A fifth of everyone older than 40 will develop heart failure. Every year, the affliction causes 3.6 million hospitalisations and more than 300,000 deaths in the US, at an overall cost to healthcare of about $35 billion. Only half of the six million or so patients living with the condition can be managed effectively through medication. The vast majority of the remainder – those who are non-responsive to drug therapy, but not sick enough to warrant invasive surgical treatment – suffer from chronic fatigue, difficulty breathing, and painful swelling while performing normal activities.
The only treatments available to most of these patients are heart transplants, which are limited by scarcity, and surgically implanted pumps called ventricular assist devices (VADs), which are so risky, invasive and expensive that they are only used as a last resort. Together, these techniques reach approximately 6,000 patients in the US every year, leaving more than two million people with no safe and effective treatment options, and no choice but to suffer a slow decline in quality of life.
There might be a safer alternative. Houston-based medical device firm, Procyrion, is developing the world’s first catheter-deployed circulatory assist device for long-term use. The Aortix, which was conceived by cardiologist
Dr Reynolds M Delgado, medical director of mechanical support devices in heart failure at the Texas Heart Institute, could replace high-risk surgical devices and lengthy hospital stays with a low-risk outpatient cardiology procedure. It is hoped it will not only improve the quality of life for millions of chronic heart failure patients, but also benefit hospitals and payers by cutting treatment costs and readmission rates.
Mend a broken heart
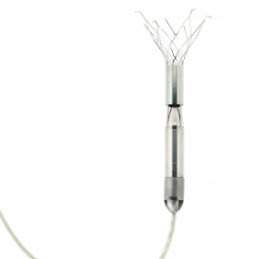
The tool is built around a powerful micro-motor mounted on a set of expandable struts. It is designed to rest and heal the heart by reducing afterload and improving blood flow to vital organs. Unlike other circulatory assist devices that involve invasive, high-risk procedures, the product is small enough – 6mm wide and less than 6.5cm long – to be deployed without surgery and with minimal risk.
Procyrion says the device could be inserted in a simple, 30-minute procedure. A cardiologist would deliver the circulatory assist device through a catheter in the femoral artery to the descending thoracic aorta (a strategic location downstream of the heart that allows for combined benefit to the heart, kidneys and other vital organs).
Once the catheter sheath is in place, it will be retracted to deploy the self-expanding nickel-titanium struts that anchor the pump to the aortic wall. This reversible anchoring is essential to Aortix’s suitability for long-term use in a walking, active patient.
Additionally, the pump’s location eliminates the common VAD risks of damage to the heart and thrombotic stroke. In contrast to traditional devices, where failure is often fatal, native blood flow is not obstructed, and a malfunction is not life-threatening.
Designed to augment the natural function of the heart by accelerating a portion of the body’s native blood flow within the micro-pump, the Aortix pushes it downstream in jets that entrain the flow bypassing the pump. In preclinical studies, the product increased the amount of blood the heart pumps by 10-15%, while simultaneously lowering its energy needs by 40%. The end result is the heart working at a sustainable level, while providing healthy blood flow and pressure to organs.
In order to design Aortix as a first-in-class blood pump, Procyrion focused on taking theories that were proven in other applications, such as stent anchoring, or fluid entrainment, and combining them with advances in motor technology.
Over the course of three years, the medical device company worked closely with motor specialists, Maxon Motor, to develop a custom motor suitable for such a demanding application.
Procyrion selected a custom-designed, high-powered EC6. Its contract manufacturer then developed this brushless DC component as a way to balance generating enough torque to drive an impeller at up to 30,000rpm, reducing heat output to minimise damage to circulating blood, and prolonging battery life. Similarly, material choices for custom electrical leads, motor housings, bearing assemblies, and speciality coatings were all made based on achieving biocompatibility while optimising durability.
Meanwhile, Procyrion worked to adapt jet pumping, commonly used in industrial applications, such as mining and refrigeration, to promote mixing and flow, to pump blood safely. Because this technique was novel to medical applications, years of simulation and pre-clinical research were focused on designing an entrainment configuration that would provide maximum flow without damaging blood cells.
Keep on pumping
In its current configuration, Aortix is powered through a flexible, transdermal lead that attaches to a pocket-sized micro-controller. The system can operate for more than eight hours on a single charge, but external battery packs and motor controllers are both hot-swappable, meaning patients could charge or replace batteries without risking pump failure.
With preclinical trials showing promising results and a first-in-man study scheduled for later this year, Procyrion is continuously investing in Aortix as a much broader platform technology. The operating efficiency and low power requirements of the device have allowed the company to design a version that uses transcutaneous energy transfer. This system enables a fully implanted, wirelessly charged battery that makes the power lead exiting the skin unnecessary and significantly reduces the risk of infection, a common side effect with other heart pumps.
Other versions of the pilot device include a magnetically coupled torque drive that spins an impeller suspended between two bearing surfaces. Although this magnetic coupling is typically used in much larger oilfield pumps, Procyrion and Maxon collaborated to successfully miniaturise it for use with Aortix. This configuration allows complete hermetic sealing of the motor, eliminating the risk of blood entering the motor core.
In addition to addressing chronic heart failure in adults, Procyion aims to adapt its catheter-deployed circulatory assist device to meet the needs of children with congenital heart defects. In April 2014, the National Capital Consortium for Pediatric Device Innovation, an FDA-sponsored grant organisation, awarded the organisation $50,000 to develop a solution for patients with single ventricle physiologies.
The current treatment for these univentricular heart defects (which occur in one in 500 births) is a series of open-heart surgeries that result in a single ventricle pumping blood throughout the entire body. As these children outgrow the capabilities of their rescued hearts, they inevitably develop severe health issues including reduced cardiac output, limited activity tolerance, stunted growth, and a significantly increased risk of developing heart failure.
Kids’ stuff
Adapting a product meant for adults to a paediatric indication is a significant challenge. One fundamental difference is the location and geometry of the paediatric configuration.
While Aortix augments left ventricular function, this paediatric pump would work in a similar role for the right ventricle: to support the return of blood from the body to the lungs.
Different blood vessel characteristics, deployment routes, and hemodynamic conditions all require consideration when addressing this new indication. In this context, Maxon saw an opportunity to further miniaturise their motors, resulting in a custom, 4mm-diameter micro-motor that allows significantly less invasive deployment and retrieval.
Procyrion intends to use its family of pumps as bridge-to-transplant, bridge-to-haemodynamic stability, or bridge-to-surgery therapies and aims to increase patient quality-of-life by resting the heart, improving organ function, and restoring mobility. In the near future, the organisation also hopes to offer a broad range of minimally invasive circulatory support devices that provide safe and effective treatment options where none currently exist. It believes treatment of heart failure in younger and healthier patients will allow for intervention before years of progressive damage occur.






