Utilizing 3D printing rather than traditional injection molding approaches for monolithic parts allows for new, porous designs to be chosen to allow for increased cell infiltration, and ultimately better wound closure. These parts may be manufactured via Fused Filament Fabrication (FFF) from Poly-Med’s portfolio of 1.75 mm 3D printing filaments, or our novel Photoset® liquid resin material that can be utilized in stereolithography (SLA) or digital light processing (DLP) 3D printing.
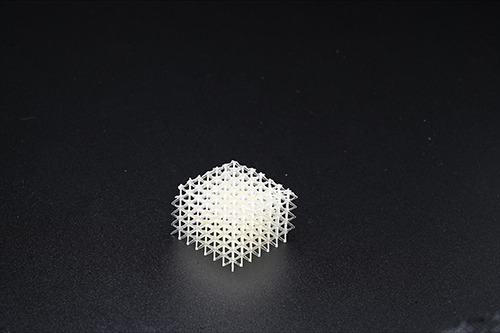
Poly-Med, Inc. offers in-house 3D printing of Photoset® resin-based 3D printing of our Photoset® material via stereolithography (SLA) or digital light processing (DLP) approaches and of 1.75 mm extruded filaments via Fused Filament Fabrication (FFF).
Analytical Testing:
Poly-Med provides in-house analytical testing that limits 3rd-party vendor touchpoints, resulting in faster speed to market during the product development cycle. In-house analytical testing for absorbable components and finished medical devices also simplifies a product’s manufacturing supply chain.
Quality:
Poly-Med, Inc. is FDA-registered, has an ISO 13485 compliant QMS, and operates a manufacturing facility in compliance with 21 CFR Part 820, Quality System Regulation. Because Poly-Med can see a product from design to manufacture, our quality assurance standards are ingrained into the very core of our company; the organizational structure, responsibilities, procedures, processes, and resources used.

