The company’s flagship Vivos DNA oral appliance, the Vivos mRNA oral appliance, and the Vivos mmRNA oral appliance have secured the FDA clearance
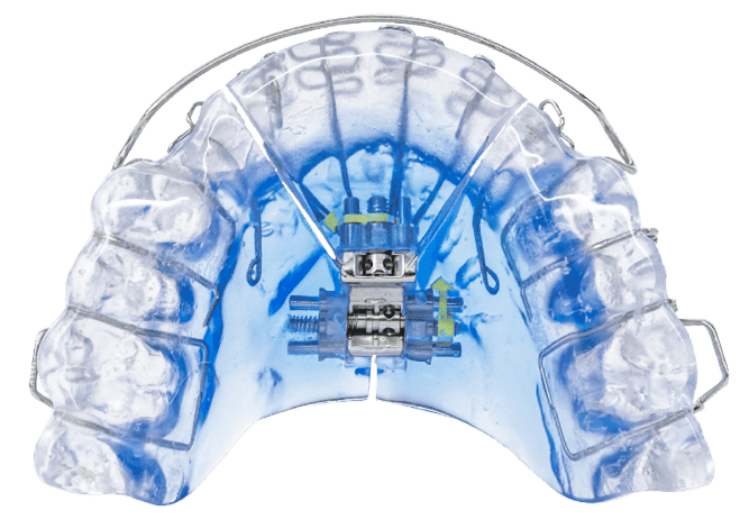
Vivos DNA appliance. (Credit: GlobeNewswire/Vivos Therapeutics, Inc)
Vivos Therapeutics has received 510(k) clearance from the US Food and Drug Administration (FDA) for Vivos’ removable CARE (Complete Airway Repositioning and/or Expansion) oral appliances for the treatment of severe obstructive sleep apnoea (OSA).
The US-based medical device and technology company is focused on the development and marketing of diagnostic and treatments for sleep related breathing issues.
Vivos Therapeutics’ method is said to be the first clinically effective nonsurgical, noninvasive, and nonpharmaceutical solution for mild to severe obstructive sleep apnoea treatment.
The treatment regimen makes use of the proprietary Vivos CARE appliance, which is intended to modify the size, form, and orientation of the soft tissues that make up a patient’s palate and/or upper airway.
According to the medical device firm, the appliances include its flagship Vivos DNA oral appliance, the Vivos mRNA oral appliance, and the Vivos mmRNA oral appliance.
The latest clearance follows the FDA 510(k) clearance for the Vivos DNA oral appliance given in January 2023 to treat mild-to-moderate OSA.
Vivos Therapeutics chairman and CEO Kirk Huntsman said: “This achievement is a pivotal milestone for Vivos and elevates our proven treatment options right into the mainstream of sleep medicine.
“It is even more important for the millions of severe OSA patients who are desperate for an effective alternative treatment.
“Before this, severe OSA patients’ only realistic treatment options were CPAP, neurostimulation implants or other invasive surgeries.
“Today, they have what we believe is a far more desirable option that is very affordable and doesn’t require surgery or a lifetime of nightly use and intervention.”
The FDA clearance for Vivos’ removable CARE oral appliances was based on the statistically significant data from 73 patients with severe obstructive sleep apnoea. The study revealed that 80% of the patients witnessed an improvement of at least one classification or a minimum of 50% improvement in the Apnea Hypopnea Index (AHI).
The mean duration of therapy was about 9.7 months, the medical device company added.
Furthermore, patients with severe sleep apnoea had better treatment outcomes than those with mild to moderate cases.
