The plating systems are designed to treat a range of deformity, trauma, and degenerative conditions to the forefoot, midfoot, rearfoot, and ankle
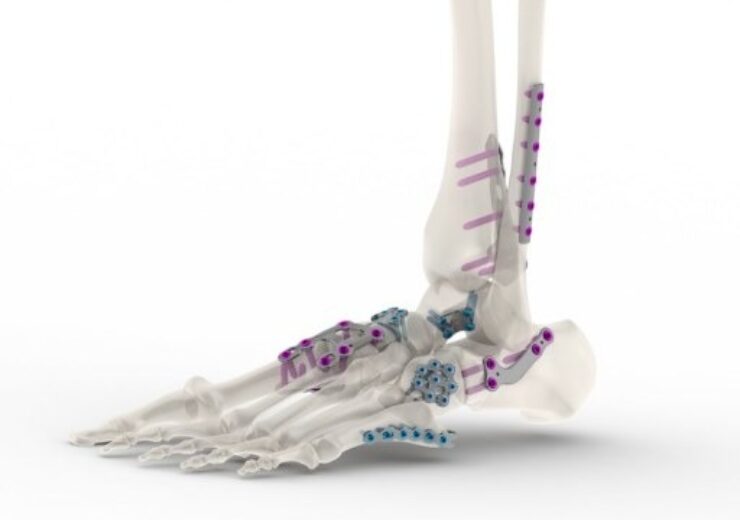
Tyber Medical has secured FDA 510(k) clearance for foot and ankle plating systems. (Credit: PRNewswire / Tyber Medical, LLC)
Orthopaedic device manufacturer Tyber Medical has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its new line of foot and ankle plating systems.
The FDA approval is applicable for more than 42 different indication-specific anatomical plating families.
Tyber Medical has developed new products using a combination of CT scans, cadaveric labs, as well as consultation with surgical thought leaders.
The company’s plating systems have been developed to treat a range of deformity, trauma, and degenerative conditions to the forefoot, midfoot, rearfoot, and ankle.
Tyber Medical plans to launch the first phase of plates in the first half of 2021
It plans to introduce the first phase of plates in the first half of 2021, as well as actively pursuing the CE mark in the second half of the same year.
The company’s foot and ankle plating systems include a 30-degree, multi-angle and locking mechanism optimised for enhanced stability between the plate and screws.
According to the company, a modular, tray delivery system can be customised with a colour-coded and clean layout, which is consistent with the surgical flow to meet surgeons’ instrumentation requirements.
The systems comprise low profile plates to reduce soft tissue irritation and maximise the variable locking technology to minimise screw head prominence.
In addition, the sterile and non-sterile plates comprise an engineered combination of material and surface modification which significantly enhances fatigue resistance.
Its plating systems for fore, mid, hindfoot, ankle repair, and ankle fusion are designated for use in the fixation of phalanges, metatarsals, and small bone fragments in the forefoot, and medium-to-large bones and multi-fragments in the mid- and hindfoot.
The 42 different indicated plates are said to stabilise fractures, joint fusions, osteotomies, nonunions, malunions, reconstruction of small bones, revision surgeries, and re-plantations to enable healing in adult patients.
Tyber Medical’s ankle fracture system is suitable for use in the fixation of fractures of the distal tibia, intra and extra-articular, and medial malleolar fractures.
Tyber Medical CEO and president Jeff Tyber said: “We are excited to receive the FDA clearance that officially launches Tyber Medical into the orthopaedic plating arena.
“Our team has developed a robust method linking intrinsic design and ease of use for a variety of surgical indications. We expect to expand this methodology beyond the lower extremities and into all areas of the skeletal anatomy.”
In August 2016, Tyber Medical expanded its product portfolio with the introduction of new lateral plating system.
