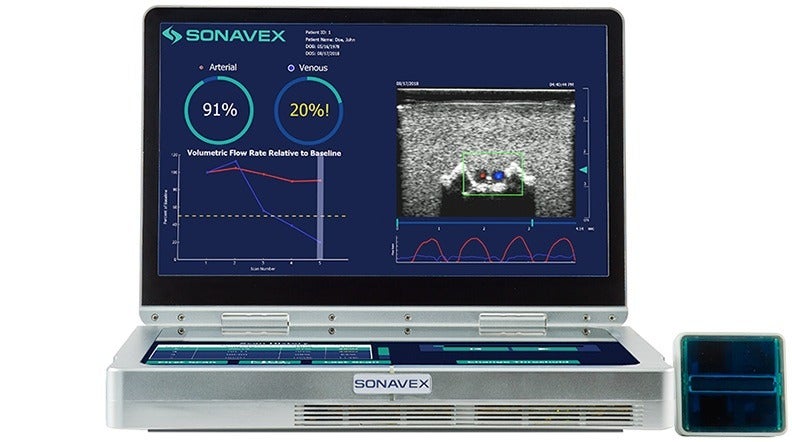
To automate visual and quantitative blood flow monitoring after surgery, the EchoSure system of Sonavex combines 3D ultrasound imaging with deep learning algorithms.
Coupled with FDA-cleared EchoMark bioresorbable markers from Sonavex, EchoSure claims to eliminate the need for ultrasound expertise to measure blood flow. The EchoSure app allows surgeons to monitor patients remotely from their mobile devices.
According to the company, EchoSure helps to avoid false-positives with real-time visualization of blood flow through the vein and artery.
Sonavex chief medical officer and president Devin O’Brien Coon said: “For decades, the surgical community has sought a simple, fast and non-invasive way to accurately quantify blood flow after microvascular and vascular surgeries.
“Putting ultrasound technology in the hands of bedside nurses for the first time may enable detection of vascular compromise earlier than clinical observation alone, providing opportunities for more rapid intervention and improved patient outcomes.”
In June 2018, Sonavex received FDA clearance for its EchoMark and EchoMark LP tissue markers. EchoMark is used for marking soft tissue sites in surgical patients. The implant’s ultrasound reflective properties and 3-dimensional shape allows surgeons to precisely mark surgical sites for clear ultrasound visualization during critical follow-up evaluations. The product is the first in the company’s portfolio to secure FDA clearance.
Sonavex CEO David Narrow said: “The Sonavex team worked tirelessly for more than five years to create a solution that enables surgeons and nurses to easily collect vital information in real-time so they can provide the best results for their patients.
“This is a critical milestone in our mission to bring transformative ultrasound technologies to patients, and we are excited to ramp up commercialization efforts this spring.”
With FDA clearance, Sonavex is planning to introduce EchoMark and EchoSure for clinical use across the US.
Sonavex is a Baltimore-based medical device company that is originally spun out of Johns Hopkins.






