The new 50mm device and the 45mm Lariat-RS are currently commercially available for the customers in Europe.
According to the company, the Lariat-RS is the only non-implant solution for complete and permanent exclusion of the LAA in Europe, making it to differentiate from all other percutaneous LAA closure devices in the region.
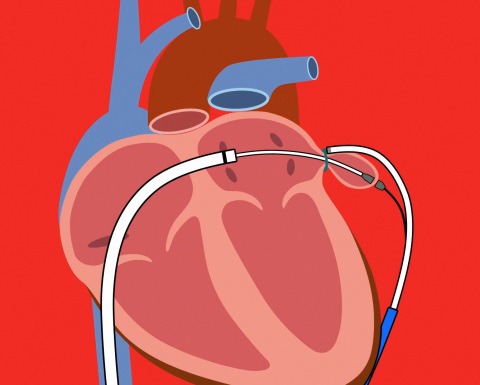
Lariat uses fluoroscopic and echocardiographic imaging guidance to help clinicians to accurately deliver a pre-tied suture loop to the base of the LAA from the outside, which leaves no metal or foreign material inside of the heart.
The LAA will disappear over time and not be a source for blood clots in patients with atrial fibrillation.
The LAA is identified as the primary source of stroke-causing blood clots originating from the heart in patients with non-valvular atrial fibrillation, said the company.
The closure of the LAA is believed to reduce the incidence of stroke and eliminate the need for life-long anticoagulation.
SentreHEART is currently carrying out aMAZE prospective, multi-center, randomized and controlled trial in around 65 centers within the US to assess the efficacy of Lariat procedure.
The trial has been designed to demonstrate the Lariat procedure for LAA closure, when used in adjunct with subsequent pulmonary vein isolation (PVI) catheter ablation, will help reduce incidence of recurrent AFib compared to PVI alone in those patients that suffer from drug-refractory, persistent and long-standing persistent AFib.
The aMAZE trial seeks to potentially treat an underlying disorder of AFib by mechanically and electrically isolating the base of the LAA in a single step using the percutaneous and non-implant Lariat suture delivery device, unlike LAA implant solutions for AFib.
Studies have showed that LARIAT can isolate electrical activity within the LAA, in addition to closing the LAA mechanically.
SentreHEART also noted that a non-implant option that can both electrically and mechanically isolate the LAA is a significant addition to the treatment armamentarium for clinicians treating patients with persistent or longstanding persistent AFib.



