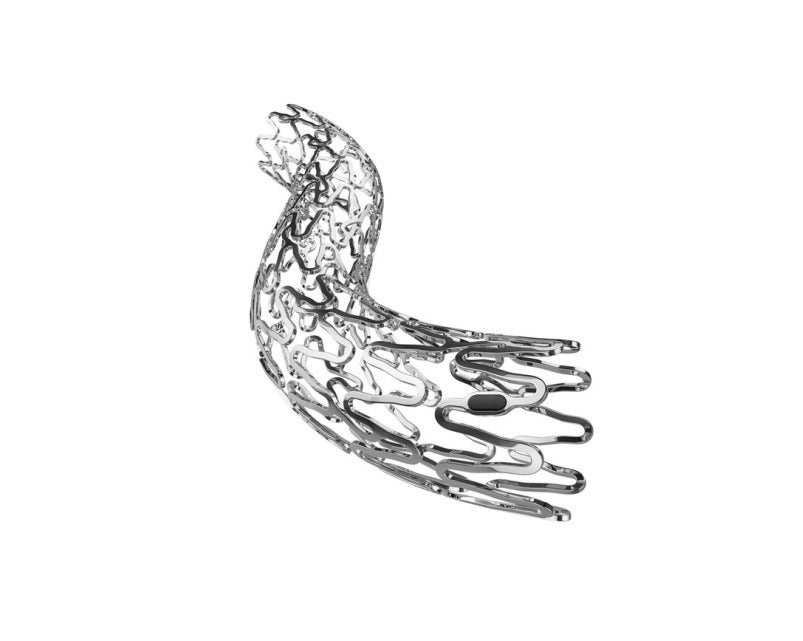
QualiMed Innovative Medizinprodukte, a wholly-owned subsidiary of Q3 Medical Devices, has secured CE mark approval for its UNITY-B percutaneous balloon expandable biodegradable biliary stent (BEBS) for biliary applications.
The percutaneous biodegradable metal alloy based stent implant has been designed for the percutaneous treatment of hepatobiliary obstruction requiring large opening drainage.
QualiMed’s UNITY-B BEBS will help eliminate removal procedures associated with traditional non-degradable implants.
The UNITY-B biodegradable percutaneous stent is developed as an alternative to eliminate the second removal procedures required with the traditional covered and uncovered metallic stents.
The large diameter balloon expandable biodegradable implant will help gastroenterologists, surgeons, and interventional radiologists to minimise the total cost of care and complications associated with the older generation of non-biodegradable plastic and metallic implants.
Q3 CEO Eric Mangiardi said: “The CE mark approval of the UNITY-B Percutaneous Biodegradable implant represents yet another milestone for QualiMed and all the companies of Q3 Medical and our collective mission of Creating Value By Helping People.
“In these uncertain times, being able to provide total cost of care cost-saving using advanced, clinically proven products is more important than ever.”
QualiMed has designed the UNITY-B biodegradable stent using the functionality of musculoskeletal system (bone and muscle).
The skeletal (Magnesium) portion of the system acts as the main support structure, while the muscle (polymer) enables to support movement and stability to address the short comings observed in first generation biodegradable technology.
According to the company, the UNITY-B biodegradable system demonstrated no adverse events and serious adverse events in a recently completed safety and efficacy clinical trial.
Mangiardi said: “As we continue the development of additional biodegradable technologies to expand our portfolio for use in the gastrointestinal tract and peripheral vasculature , we are striving to shift the paradigm for treatments by reducing complications and eliminating the need for additional removal procedures or latent complications associated with the current treatment options.”
Founded in 1997 as an OEM manufacturer for implantable medical devices, QualiMed recently started developing various biodegradable technologies, drug device combination products, and micro-intervention implants.


