Zephyr system is claimed to be the first minimally-invasive device approved in the US to treat severe emphysema, a progressive and life-threatening form of chronic obstructive pulmonary disease (COPD).
The FDA has approved the device based on positive clinical data from the pivotal Liberate study and two other multicenter randomized control trials.
According to the company, the patients treated with Zephyr Valves were able to breathe easier in the Liberate study and showed a significantly improved quality of life compared to patients who received medical management alone.
Liberate study lead investigator Dr Gerard Criner said: “Zephyr Valves are a major step forward in treating severe emphysema patients who consistently feel short of breath despite all the medications we can offer. I have seen Zephyr Valve-treated patients getting back to a more active life doing the things they enjoy.”
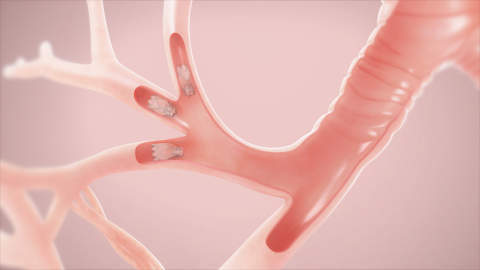
Tiny Zephyr valves are placed in the airways to occlude a diseased part of the lungs and reduce hyperinflation during a bronchoscopic procedure requiring no cutting or incisions.
The process will help the healthier parts of the lungs to expand and lifts pressure off the diaphragm, enabling to decrease shortness of breath and make breathing easier.
Pulmonx Chartis pulmonary assessment system and StratX lung analysis platform will allow the physicians to detect potential responders to Zephyr valve treatment.
Pulmonx Zephyr endobronchial valves are implantable bronchial valves used for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation.
Pulmonx CEO Glen French said: “We thank FDA for its swift review of the Zephyr Valve. By combining the Zephyr Valves and our patient selection tools, we are bringing precision medicine to the treatment of severe emphysema.”




