The new indication enables Pipeline Flex embolization device to treat patients with small or medium, wide-necked brain aneurysms in the territory from the petrous to the terminus of the internal carotid artery.
Earlier, the device is indicated for the endovascular treatment of adults with large or giant wide-necked intracranial aneurysms (IAs) in the internal carotid artery from the petrous to the superior hypophyseal segments.
The latest approval was based on clinical data from the prospective study on embolization of intracranial aneurysms with the Pipeline device (PREMIER) trial.
Medtronic has studied 141 subjects with a mean aneurysm size of 5.0±1.92 mm in the trial.
According to the company, the data demonstrated one-year occlusion rates of 76.7% with the use of 1.1 device per subject on average and 2.2% occurrence of major stroke or neurological death.
PREMIER trial principal investigator Dr Ricardo Hanel said: “PREMIER is another landmark study with Pipeline and moves the bar on the safe treatment of wide-necked brain aneurysms.”
In the US, Pipeline Flex device is advised for use for the endovascular treatment of complex wide-necked intracranial aneurysms located in the ICA and attached to parent vessels measuring between 2.0 and 5.0 mm in diameter.
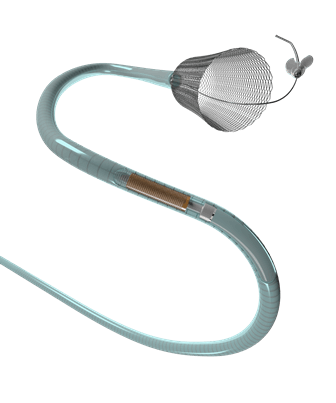
Pipeline Flex embolization device, which was developed to divert blood flow away from an aneurysm, is provided with a braided cylindrical mesh tube that is implanted across the base or neck of the aneurysm.
Pipeline Flex device will halt the blood flow to the aneurysm and reconstruct the diseased section of the parent vessel.
The FDA approval for the device has been achieved after completing the Pipeline for Uncoilable or Failed aneurysms (PUFs) trial, which is a five-year study for large and giant wide-necked aneurysms of the intracranial internal carotid artery (ICA).
Medtronic restorative therapies group’ neurovascular business general manager and vice president Stacey Pugh said: “Working hand-in-hand with physicians to develop new technology and clinical data is at the core of our mission.
“The PREMIER study not only demonstrated excellent safety and efficacy outcomes but also delivered on our commitment to broadening access to innovative therapies for new groups of patients requiring aneurysm treatment.”






