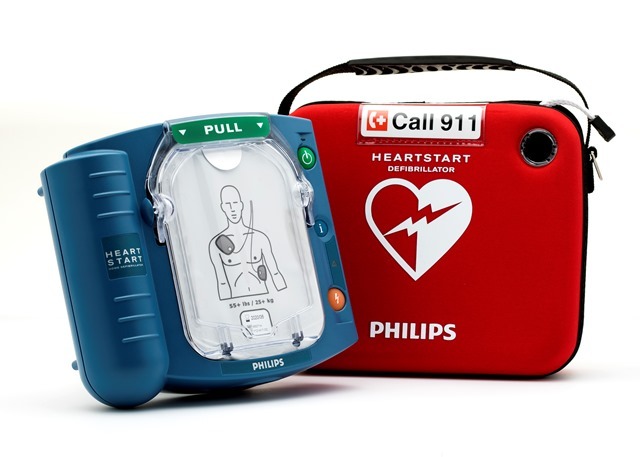
Philips’ HeartStart OnSite and HeartStart Home defibrillators are the only over-the-counter automated external defibrillators (AEDs) available to consumers in the US, while the HeartStart Home defibrillator is the only AED specifically indicated for home environments.
“We are committed to delivering high quality, innovative AEDs to provide personalized therapy to victims of sudden cardiac arrest,” said Arman Voskerchyan, Business Leader Therapeutic Care at Philips.
“Premarket approval for our HeartStart OnSite and HeartStart Home defibrillators, currently the only over-the-counter AEDs available in the US, reflects the robust work of our teams that delivered the strong and extensive technical, clinical and production data included in the PMA filing for these devices.”
Royal Philips is a leading health technology company focused on improving people’s health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care.
Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care.
Source: Company Press Release






