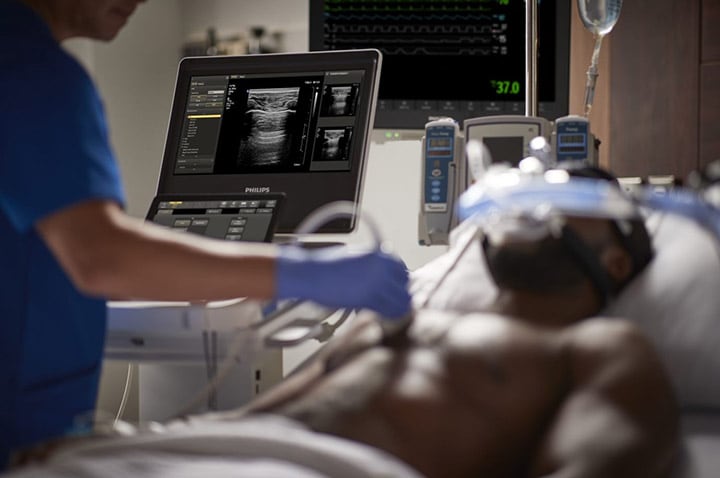
Dutch health technology company Philips has secured the US Food and Drug Administration 510(k) approval for its latest compact ultrasound system, the 5000 Compact Series.
The new Ultrasound 5000 Compact Series leverages the latest advancements in the company’s ultrasound technology to deliver cart-based premium image quality in compact form.
It features various diagnostic ultrasound solutions and addresses the needs of several clinical specialities, including point of care, cardiology, general imaging, and obstetrics and gynaecology.
Designed to provide clear images that rapidly inform and enable diagnosis confirmation, the solution is said to prevent the need for costly, time-consuming repeat scans.
The device comes with a wide range of clinical capabilities, including real-time collaboration, and wireless connectivity and reporting, said the company.
Philips point-of-care ultrasound general manager Matthijs Groot Wassink said: “Globally, clinicians are faced with increasing pressures to deliver higher patient throughput for quick, confident diagnoses via ultrasound exams, while receiving more and more requests to perform rapid ultrasound exams at the bedside.
“Until now, portable ultrasound systems have typically meant decreased performance, lack of advanced features, and lower grade image quality.
Philips designed the new Ultrasound 5000 Compact Series by combining portability with performance, leveraging its experience in ultrasound imaging.
It enables clinicians to benefit from its compact design, without compromising on performance, delivering clinical decision support with mobile efficiency.
Based on its real-time collaboration and remote consultations and training, and integrated connectivity features, the system enables clinicians to make the right diagnosis the first time.
The compact ultrasound solution shares the same user interfaces, features, touchscreens, transducers, and workflows as its EPIQ and Affiniti ultrasound systems, said Philips.
Wassink added: “With this clearance for our next generation portable Philips Ultrasound 5000 Compact Series, clinicians now have the ability to make quicker and more confident diagnoses.
“Patients within locations such as hospitals, surgery centres, clinics, physician offices, and point-of-care settings, also have faster access to quicker results, physician communication and ultimately, increased peace of mind in their treatment plans.”






