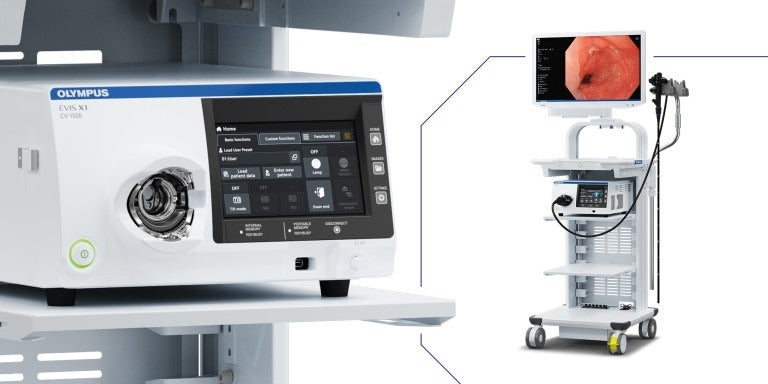
Olympus has received the US Food and Drug Administration (FDA) clearance for its new EVIS X1 endoscopy system in addition to two compatible gastrointestinal endoscopes, which are the GIF-1100 gastrointestinal videoscope and the CF-HQ1100DL/I colonovideoscope.
The GIF-1100 gastrointestinal videoscope has been cleared to be used within the upper digestive tract including the stomach, oesophagus, and duodenum.
The CF-HQ1100DL/I colonovideoscope has been authorised to be used within the lower digestive tract. This includes the rectum, anus, sigmoid colon, colon, and ileocecal valve.
According to the medical technology company, the Olympus GI endoscopy systems can help diagnose, treat, and monitor diseases and disorders of the upper and lower GI tract. The endoscopy systems cover Crohn’s disease, Celiac disease, colorectal cancer, acid reflux, and ulcers.
Olympus said that the introduction of new imaging technologies can support doctors in visualising abnormalities during colonoscopy screening.
Olympus America Medical Systems Group president Richard Reynolds said: “We are thrilled that we will soon be able to bring this new endoscopy system to physicians and their patients in the US.
“As a leading medical technology company, Olympus strives to offer physicians the most advanced technologies for minimally invasive procedures such as GI endoscopy.”
The EVIS X1 endoscopy system has three new technologies to aid physicians in visualising GI bleeds and anatomical structures.
To enable the three new enhancements, Olympus has replaced the Xenon bulb in the EVIS EXERA III system with five LEDs that can generate other light combinations along with the white light.
EVIS X1, which was launched in April 2020, has Red Dichromatic Imaging (RDI) technology for optical-digital observation with the help of green illumination light and red dichromatic narrow-band light.
The second new technology is Texture and Colour Enhancement Imaging (TXI) technology, which has been designed to correct the brightness of dark areas. It is also designed to highlight tonal changes, patterns, and image outlines.
The third technology in the endoscopy system is the Brightness Adjustment Imaging with Maintenance of Contrast (BAI-MAC) technology. It is designed to sustain the brightness of the bright part and correct the brightness of the dark part of the endoscopic image, the medical technology company added.
NBI, RDI, TXI, and BAI-MAC technologies are not meant to replace histopathological sampling as a means of diagnosis, Olympus said.



