NinePoint Medical has secured approval from the US Food and Drug Administration (FDA) for its NvisionVLE imaging system for pancreatic and biliary applications.
With the approval, the NvisionVLE system can be used in the pancreas and bile duct. The new anatomical indications are an extension to the earlier existing esophageal applications of NinePoint’s optical coherence tomography (OCT) imaging platform.
NinePoint Medical president and CEO Dr Eman Namati said: “Our group over the years has really pushed the limits of engineering innovation to help patients.
“This past year has been especially fruitful, with the clearance and market launch of first-of-its-kind artificial intelligence technology, and now with the clearance of a pancreatico-biliary application for the Low-Profile Optical Probe.”
NvisionVLE imaging system
NvisionVLE imaging system is claimed to be the first FDA-cleared artificial intelligence imaging product in gastroenterology, which assists in image review and creation of real-time superficial laser marks to guide tissue acquisition.
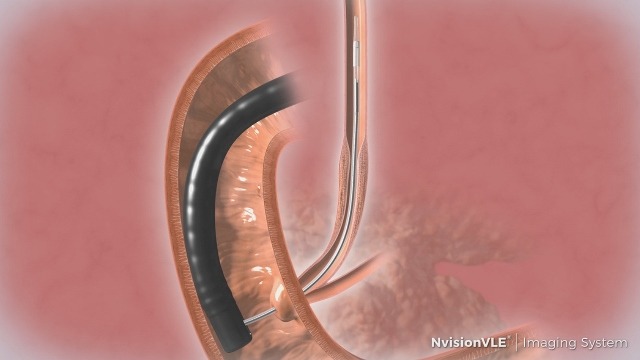
The physicians can use the NvisionVLE imaging system to capture real-time and high-resolution volumetric images of the tissue surface and subsurface. It enables gastroenterologists to better assess tissue for regions of interest, which cannot be seen with other medical imaging technologies.
The newly cleared application is linked with NinePoint’s Low-Profile Optical Probe, a 7 French diameter imaging probe developed to deal with handle small and tortuous anatomies observed in the pancreatico-biliary system.
NvisionVLE can be used as an imaging tool in the assessment of human tissue microstructure, including esophageal tissue microstructure. It offers two-dimensional, cross-sectional and real-time depth visualisation, as well as helps to mark areas of tissue.
The latest approval is said to be another significant step for the strategic collaboration between NinePoint Medical and their exclusive worldwide distributor, Merit Medical Systems.
Merit Medical chairman and CEO Fred Lampropoulos said: “NinePoint continues to produce innovative and high quality products that our team has been very impressed with.”
In NinePoint Medical has secured approval from the FDA for Intelligent Real-time Image Segmentation (IRIS) software upgrade for the NvisionVLE imaging system.






