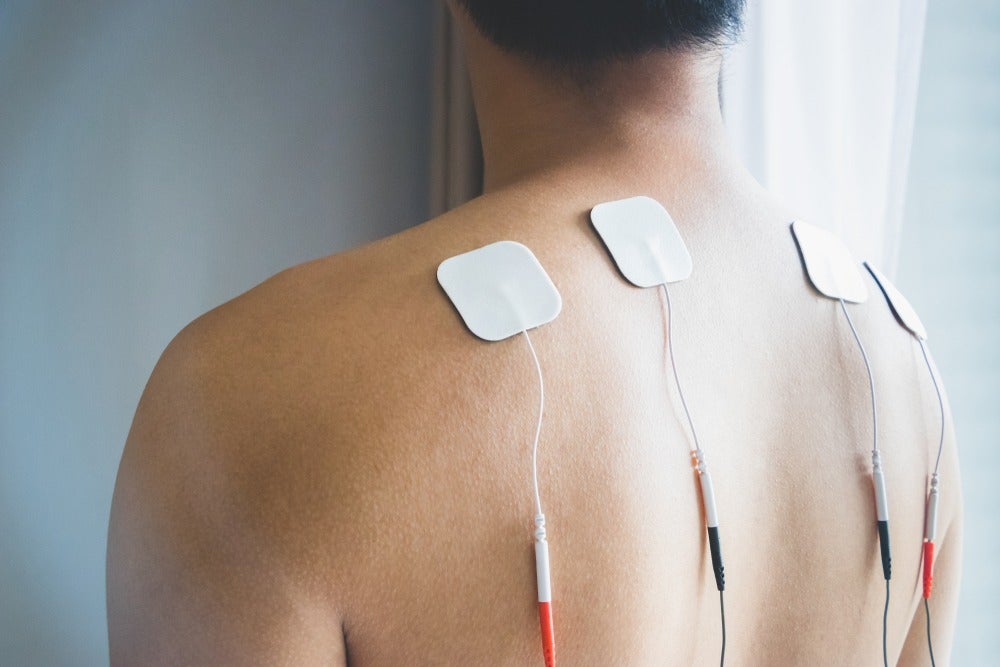
Constant developments and novel breakthroughs in the neurostimulation devices market are helping provide pain management to a growing number of individuals affected by psychiatric and neurological conditions.
Also known as neurostimulators, these devices are improving the quality of life of those suffering from profound losses to various sensory organs.
Neurostimulation technology is also helping patients in reduction of severe, chronic pain which would require expensive, round-the-clock, high-dose opioid therapy.
One of the key reasons the technology has become one of the most preferred treatment modalities is because it is patient centred.
The proven efficacy and growing acceptance of neurostimulators in modern medicine is driving the market for neurostimulation devices, which is set to reach a value of more than $22bn globally by 2026, claims Global Market Insights.
Robust potential in pain management applications
Chronic pain, which affects a vast number of geriatric people, trauma and surgery patients, as well as those undergoing different therapies, has been linked to numerous physical and mental conditions.
It contributes to lost productivity and high healthcare costs and is among the most common reasons adults seek medical assistance.
It’s also been linked to restrictions in daily activities, anxiety and depression, dependence on opioids, and a poor quality of life.
Opioids are largely prescribed as a long-term pain management solution, but many countries have witnessed increased opioid addiction.
In the U.S., for example, the Covid-19 pandemic has led to an uptick in opioid abuse, with at least 40 states reporting a higher risk of opioid-related deaths since the start of the pandemic.
Neurostimulation could be a safer and more efficient solution for alleviating chronic pain and should experience widespread adoption in the near future.
Numerous biotech companies have also launched neurostimulators to help opioid addicts overcome pain and other withdrawal symptoms.
For example, in April 2021, Spark Biomedical, a leading medical device company had rolled out a new neurostimulation device called Sparrow Therapy System to help opioid addicts work through withdrawal symptoms.
The device uses low doses of electricity to stimulate endorphin production, which helps to alleviate the pain and fear that patients experience during withdrawal.
The Sparrow Therapy System recently received FDA approval to reduce opioid dependence.
Neurostimulation for back pain management
Among all types of chronic pain, back pain is the second leading reason for hospital visits and among the major reasons for hospital admission or surgeries.
According to the US Centers for Disease Control and Prevention (CDC), between 60% and 80% of people experience some form of lower back pain during their lifetime.
Earlier this year, Mainstay Medical commercially launched ReActiv8, an implantable neurostimulation device for chronic low back pain (CLBP), in Australia.
The device can potentially be helpful to adults with intractable CLBP linked with the dysfunction of the lumbar multifidus, a key stabilising muscle in the lower back.
Neurostimulation technology, owing to its improving safety, reliability, and efficiency, will continue to find new applications in pain management.
Back in 2019, pain management applications dominated the neurostimulation devices industry with more than 70% market share.
Rising burden of Parkinson’s disease in the US
Did you know that nearly one million individuals in the US are living with Parkinson’s disease? By 2030, this number is estimated to reach nearly 1.2 million.
The combined direct and indirect cost of its treatment, including lost income and social security payments is significantly high.
The arrival of new devices based on neurostimulation could prove to be an effective solution for people living with Parkinson’s disease.
Numerous brain stimulation technologies for treatment of the disease have been launched in recent years.
In January 2021, for instance, Boston Scientific’s latest deep neurostimulator Vercise Genus received FDA approval for application in Parkinson’s disease treatment.
The technology was designed to treat the symptoms such as tremors and slowness of movement.
The Vercise Genus features Bluetooth-powered implantable pulse generators and comes in both rechargeable and non-rechargeable models.
A new neurostimulation device backed by the National Institutes of Health developed by a team of engineers at the University of California, San Francisco in May 2021 is among the first technologies to offer long-term monitoring of brain activity in Parkinson’s patients.
The neurostimulator is implanted in the patient’s chest and connected to electrodes in the brain via thin wires.
These electrodes track brain activity and transmit data to a pocket-sized device nearby, where the data is uploaded to a tablet or a cloud-based server via Bluetooth.
Wide prevalence of migraine in North America
The market for neurostimulation devices in North America generated about $4bn in revenue during 2019 and should expand substantially, considering the wide occurrence of numerous neurological conditions such as migraine, anxiety, depression, epilepsy, Parkinson’s disease, and others.
Migraine is among the leading causes of disability in people in the region up to the age of 50, affecting one out of seven individuals, most of which are women.
Many of these individuals suffer from chronic migraine, meaning they experience migraines for about 22 days per month on average.
Leading biotech and biomedical companies in the region are focusing on designing neurostimulation devices to improve the quality of life of migraine patients.
Last year in October, CEFALY Technology’s DUAL neurostimulator had received FDA clearance as an over-the-counter solution for acute and preventive treatment of migraines in adults.
The CEFALY DUAL is apparently the first dual-purpose, external Trigeminal Nerve Stimulator (eTNS) device available over-the-counter in the US for preventing migraine headaches.
Previously available only with a prescription, the FDA clearance of CEFALY DUAL will provide approximately 39 million American migraine patients over-the-counter access to the neurostimulation device.
The emerging segment of implantable medical devices has received widespread approval across the global healthcare sector.
These technologies are also witnessing traction in neurostimulation applications.
In November 2020, Salvia BioElectronics, a neurostimulation device manufacturer targeting chronic migraine, received Breakthrough Device Designation from the FDA for its implantable neurostimulator designed to address chronic migraine.
As the healthcare needs of patients living with neurological disorders increase, neurostimulation technology and devices will only continue to gain prominence.
Abbott, Medtronic, Bayer, Nevro, Laborie, IntraPace, NuroPace, Neuronetics, and SPR Therapeutics are among the prominent names in the global neurostimulation devices market.
Strong developments directed towards treating epilepsy, incontinence issues, spinal injuries and other chronic health problems will outline the overall industry trends.





